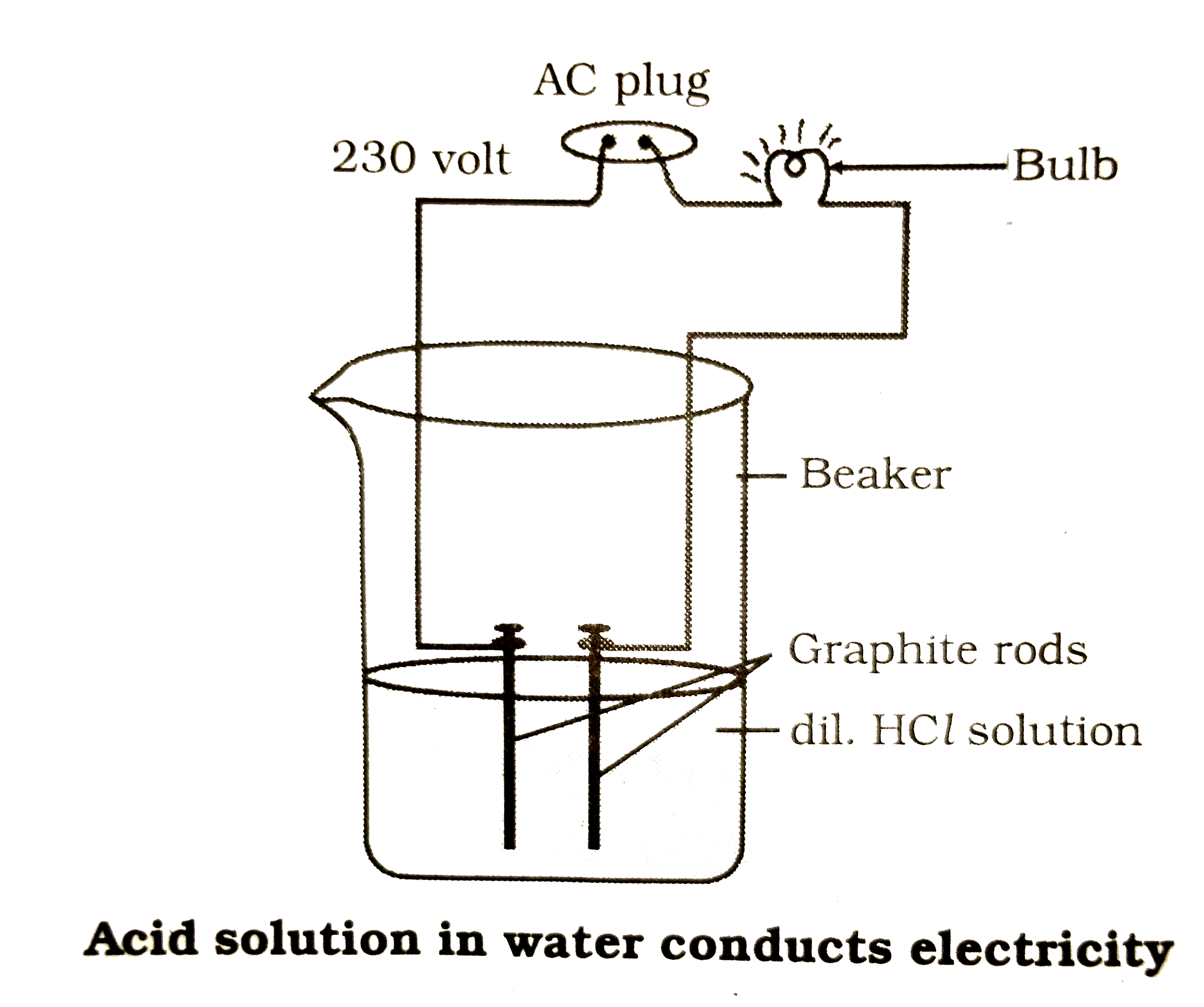Water Conduct Electricity Physics . The conductivity of water is a measure of the capability of water to pass electrical flow. The conductivity of water is a measure of the capability of water to pass electrical flow. Learn the factors affecting water conductivity and its importance here There are no ions in pure water, like fully deionized or distilled water. Interestingly, if the water contains very large amounts of solutes and ions, then the water becomes such an efficient conductor of. Pure water is an electrical insulator. But provide an ionic compound in the form of salt, and you complete the circuit. Pure water does not conduct electricity. Water’s ability to conduct electricity hinges on the presence of ion—charged particles. When salts are dissolved in water, they. Pure water doesn’t conduct electricity. Any impurities, like salts, in the water enable it to conduct electricity. This ability directly depends on the concentration of conductive. Pure water is a very poor conductor (resistivity is actually used as a measure of purity).
from www.doubtnut.com
When salts are dissolved in water, they. Learn the factors affecting water conductivity and its importance here This ability directly depends on the concentration of conductive. But provide an ionic compound in the form of salt, and you complete the circuit. Interestingly, if the water contains very large amounts of solutes and ions, then the water becomes such an efficient conductor of. Pure water is an electrical insulator. Pure water does not conduct electricity. Pure water is a very poor conductor (resistivity is actually used as a measure of purity). There are no ions in pure water, like fully deionized or distilled water. The conductivity of water is a measure of the capability of water to pass electrical flow.
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET
Water Conduct Electricity Physics The conductivity of water is a measure of the capability of water to pass electrical flow. The conductivity of water is a measure of the capability of water to pass electrical flow. Pure water is a very poor conductor (resistivity is actually used as a measure of purity). The conductivity of water is a measure of the capability of water to pass electrical flow. Learn the factors affecting water conductivity and its importance here This ability directly depends on the concentration of conductive. Pure water is an electrical insulator. Pure water doesn’t conduct electricity. When salts are dissolved in water, they. Water’s ability to conduct electricity hinges on the presence of ion—charged particles. There are no ions in pure water, like fully deionized or distilled water. Any impurities, like salts, in the water enable it to conduct electricity. Interestingly, if the water contains very large amounts of solutes and ions, then the water becomes such an efficient conductor of. Pure water does not conduct electricity. But provide an ionic compound in the form of salt, and you complete the circuit.
From www.youtube.com
5.2 Does water conduct electricity? YouTube Water Conduct Electricity Physics This ability directly depends on the concentration of conductive. There are no ions in pure water, like fully deionized or distilled water. But provide an ionic compound in the form of salt, and you complete the circuit. Any impurities, like salts, in the water enable it to conduct electricity. Learn the factors affecting water conductivity and its importance here Pure. Water Conduct Electricity Physics.
From www.teachoo.com
Why does aqueous solution of an acid conduct electricity? Class 10 Water Conduct Electricity Physics Pure water is a very poor conductor (resistivity is actually used as a measure of purity). There are no ions in pure water, like fully deionized or distilled water. Learn the factors affecting water conductivity and its importance here The conductivity of water is a measure of the capability of water to pass electrical flow. Water’s ability to conduct electricity. Water Conduct Electricity Physics.
From www.teachoo.com
Why does distilled water not conduct electricity, whereas rain does? Water Conduct Electricity Physics Learn the factors affecting water conductivity and its importance here But provide an ionic compound in the form of salt, and you complete the circuit. The conductivity of water is a measure of the capability of water to pass electrical flow. Pure water is an electrical insulator. Interestingly, if the water contains very large amounts of solutes and ions, then. Water Conduct Electricity Physics.
From lessonlibcocainises.z22.web.core.windows.net
Conducting Electricity With Salt Water Water Conduct Electricity Physics Pure water is a very poor conductor (resistivity is actually used as a measure of purity). Learn the factors affecting water conductivity and its importance here This ability directly depends on the concentration of conductive. Pure water does not conduct electricity. The conductivity of water is a measure of the capability of water to pass electrical flow. When salts are. Water Conduct Electricity Physics.
From techiescientist.com
Does Aluminum Conduct Electricity? Techiescientist Water Conduct Electricity Physics Water’s ability to conduct electricity hinges on the presence of ion—charged particles. But provide an ionic compound in the form of salt, and you complete the circuit. There are no ions in pure water, like fully deionized or distilled water. Any impurities, like salts, in the water enable it to conduct electricity. When salts are dissolved in water, they. Pure. Water Conduct Electricity Physics.
From www.youtube.com
electrical conductivity with salt water working model school science Water Conduct Electricity Physics Water’s ability to conduct electricity hinges on the presence of ion—charged particles. The conductivity of water is a measure of the capability of water to pass electrical flow. This ability directly depends on the concentration of conductive. But provide an ionic compound in the form of salt, and you complete the circuit. The conductivity of water is a measure of. Water Conduct Electricity Physics.
From ximena-has-hurley.blogspot.com
What Are Substances Called Whose Water Solutions Conduct Electricity Water Conduct Electricity Physics Pure water is a very poor conductor (resistivity is actually used as a measure of purity). Water’s ability to conduct electricity hinges on the presence of ion—charged particles. There are no ions in pure water, like fully deionized or distilled water. Learn the factors affecting water conductivity and its importance here Pure water is an electrical insulator. Interestingly, if the. Water Conduct Electricity Physics.
From www.pinterest.com
Salt Water Conductivity Experiment Middle school science experiments Water Conduct Electricity Physics There are no ions in pure water, like fully deionized or distilled water. This ability directly depends on the concentration of conductive. But provide an ionic compound in the form of salt, and you complete the circuit. When salts are dissolved in water, they. Any impurities, like salts, in the water enable it to conduct electricity. Interestingly, if the water. Water Conduct Electricity Physics.
From brainly.in
draw a neat diagram to show acid solution in water conducts electricity Water Conduct Electricity Physics Pure water is an electrical insulator. Water’s ability to conduct electricity hinges on the presence of ion—charged particles. Learn the factors affecting water conductivity and its importance here There are no ions in pure water, like fully deionized or distilled water. When salts are dissolved in water, they. The conductivity of water is a measure of the capability of water. Water Conduct Electricity Physics.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at Water Conduct Electricity Physics Pure water is a very poor conductor (resistivity is actually used as a measure of purity). The conductivity of water is a measure of the capability of water to pass electrical flow. Interestingly, if the water contains very large amounts of solutes and ions, then the water becomes such an efficient conductor of. When salts are dissolved in water, they.. Water Conduct Electricity Physics.
From www.youtube.com
Does Water Really Conduct Electricity? YouTube Water Conduct Electricity Physics Pure water is an electrical insulator. Any impurities, like salts, in the water enable it to conduct electricity. Pure water is a very poor conductor (resistivity is actually used as a measure of purity). Water’s ability to conduct electricity hinges on the presence of ion—charged particles. Learn the factors affecting water conductivity and its importance here This ability directly depends. Water Conduct Electricity Physics.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Water Conduct Electricity Physics The conductivity of water is a measure of the capability of water to pass electrical flow. But provide an ionic compound in the form of salt, and you complete the circuit. Pure water does not conduct electricity. This ability directly depends on the concentration of conductive. The conductivity of water is a measure of the capability of water to pass. Water Conduct Electricity Physics.
From dewwool.com
Conductivity of water DewWool Water Conduct Electricity Physics This ability directly depends on the concentration of conductive. Pure water is an electrical insulator. Any impurities, like salts, in the water enable it to conduct electricity. Pure water doesn’t conduct electricity. There are no ions in pure water, like fully deionized or distilled water. Pure water is a very poor conductor (resistivity is actually used as a measure of. Water Conduct Electricity Physics.
From www.youtube.com
Why Saltwater Conducts Electricity Science Experiment YouTube Water Conduct Electricity Physics Pure water is an electrical insulator. But provide an ionic compound in the form of salt, and you complete the circuit. Pure water doesn’t conduct electricity. Learn the factors affecting water conductivity and its importance here Pure water is a very poor conductor (resistivity is actually used as a measure of purity). Any impurities, like salts, in the water enable. Water Conduct Electricity Physics.
From byjus.com
Does water with the help of electrolyte conduct electricity or Water Conduct Electricity Physics But provide an ionic compound in the form of salt, and you complete the circuit. Interestingly, if the water contains very large amounts of solutes and ions, then the water becomes such an efficient conductor of. The conductivity of water is a measure of the capability of water to pass electrical flow. There are no ions in pure water, like. Water Conduct Electricity Physics.
From www.youtube.com
Electrical conductivity with salt water YouTube Water Conduct Electricity Physics When salts are dissolved in water, they. The conductivity of water is a measure of the capability of water to pass electrical flow. Interestingly, if the water contains very large amounts of solutes and ions, then the water becomes such an efficient conductor of. But provide an ionic compound in the form of salt, and you complete the circuit. Pure. Water Conduct Electricity Physics.
From byjus.com
Why does an aqueous solution of an acid conduct electricity? Water Conduct Electricity Physics Any impurities, like salts, in the water enable it to conduct electricity. The conductivity of water is a measure of the capability of water to pass electrical flow. There are no ions in pure water, like fully deionized or distilled water. Pure water does not conduct electricity. When salts are dissolved in water, they. This ability directly depends on the. Water Conduct Electricity Physics.
From www.studocu.com
1.PRAC Does Water Conduct Electricity Does Water Conduct Electricity Water Conduct Electricity Physics This ability directly depends on the concentration of conductive. Learn the factors affecting water conductivity and its importance here Pure water does not conduct electricity. But provide an ionic compound in the form of salt, and you complete the circuit. Pure water is a very poor conductor (resistivity is actually used as a measure of purity). Any impurities, like salts,. Water Conduct Electricity Physics.
From howtofunda.com
electrical conductivity experiment using saltwater Free Science Water Conduct Electricity Physics There are no ions in pure water, like fully deionized or distilled water. This ability directly depends on the concentration of conductive. Learn the factors affecting water conductivity and its importance here Interestingly, if the water contains very large amounts of solutes and ions, then the water becomes such an efficient conductor of. The conductivity of water is a measure. Water Conduct Electricity Physics.
From www.rookieparenting.com
Does Water Conduct Electricity? Simple Experiment Water Conduct Electricity Physics Interestingly, if the water contains very large amounts of solutes and ions, then the water becomes such an efficient conductor of. Pure water is an electrical insulator. Pure water does not conduct electricity. Water’s ability to conduct electricity hinges on the presence of ion—charged particles. Learn the factors affecting water conductivity and its importance here Pure water is a very. Water Conduct Electricity Physics.
From www.youtube.com
Is pure distilled water conduct electricity ? ClassX, part9 YouTube Water Conduct Electricity Physics Pure water is an electrical insulator. Pure water does not conduct electricity. There are no ions in pure water, like fully deionized or distilled water. But provide an ionic compound in the form of salt, and you complete the circuit. When salts are dissolved in water, they. Learn the factors affecting water conductivity and its importance here Pure water doesn’t. Water Conduct Electricity Physics.
From www.doubtnut.com
[Punjabi] Does pure water conduct electricity ?if not, what can we do Water Conduct Electricity Physics Interestingly, if the water contains very large amounts of solutes and ions, then the water becomes such an efficient conductor of. There are no ions in pure water, like fully deionized or distilled water. The conductivity of water is a measure of the capability of water to pass electrical flow. Any impurities, like salts, in the water enable it to. Water Conduct Electricity Physics.
From www.doubtnut.com
Why does not distilled water conduct electricity, whereas rain water does Water Conduct Electricity Physics Any impurities, like salts, in the water enable it to conduct electricity. Learn the factors affecting water conductivity and its importance here But provide an ionic compound in the form of salt, and you complete the circuit. Interestingly, if the water contains very large amounts of solutes and ions, then the water becomes such an efficient conductor of. Pure water. Water Conduct Electricity Physics.
From www.shutterstock.com
7 Salt Water Conduct Electricity Images, Stock Photos & Vectors Water Conduct Electricity Physics Any impurities, like salts, in the water enable it to conduct electricity. Learn the factors affecting water conductivity and its importance here But provide an ionic compound in the form of salt, and you complete the circuit. Pure water is an electrical insulator. Pure water is a very poor conductor (resistivity is actually used as a measure of purity). The. Water Conduct Electricity Physics.
From courses.lumenlearning.com
Conductors and Insulators Physics II Water Conduct Electricity Physics The conductivity of water is a measure of the capability of water to pass electrical flow. Learn the factors affecting water conductivity and its importance here Any impurities, like salts, in the water enable it to conduct electricity. Pure water does not conduct electricity. The conductivity of water is a measure of the capability of water to pass electrical flow.. Water Conduct Electricity Physics.
From www.slideserve.com
PPT L 26 Electricity and [3] PowerPoint Presentation ID Water Conduct Electricity Physics But provide an ionic compound in the form of salt, and you complete the circuit. The conductivity of water is a measure of the capability of water to pass electrical flow. Water’s ability to conduct electricity hinges on the presence of ion—charged particles. Learn the factors affecting water conductivity and its importance here Pure water is a very poor conductor. Water Conduct Electricity Physics.
From msestudent.com
Why Do Metals Conduct Electricity? Materials Science & Engineering Water Conduct Electricity Physics Water’s ability to conduct electricity hinges on the presence of ion—charged particles. Interestingly, if the water contains very large amounts of solutes and ions, then the water becomes such an efficient conductor of. Pure water is an electrical insulator. This ability directly depends on the concentration of conductive. But provide an ionic compound in the form of salt, and you. Water Conduct Electricity Physics.
From www.rookieparenting.com
Electricity Science Projects Water Conduct Electricity Physics There are no ions in pure water, like fully deionized or distilled water. Pure water is a very poor conductor (resistivity is actually used as a measure of purity). Pure water doesn’t conduct electricity. Water’s ability to conduct electricity hinges on the presence of ion—charged particles. The conductivity of water is a measure of the capability of water to pass. Water Conduct Electricity Physics.
From telgurus.co.uk
How do metals conduct electricity? Chemistry Questions TEL Gurus Water Conduct Electricity Physics But provide an ionic compound in the form of salt, and you complete the circuit. Water’s ability to conduct electricity hinges on the presence of ion—charged particles. Pure water doesn’t conduct electricity. Pure water does not conduct electricity. Interestingly, if the water contains very large amounts of solutes and ions, then the water becomes such an efficient conductor of. Learn. Water Conduct Electricity Physics.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at Water Conduct Electricity Physics There are no ions in pure water, like fully deionized or distilled water. When salts are dissolved in water, they. The conductivity of water is a measure of the capability of water to pass electrical flow. The conductivity of water is a measure of the capability of water to pass electrical flow. Learn the factors affecting water conductivity and its. Water Conduct Electricity Physics.
From cleanbreakrecovery.com
Does Alcohol Conduct Electricity? Water Conduct Electricity Physics Water’s ability to conduct electricity hinges on the presence of ion—charged particles. Learn the factors affecting water conductivity and its importance here Interestingly, if the water contains very large amounts of solutes and ions, then the water becomes such an efficient conductor of. Pure water is an electrical insulator. Any impurities, like salts, in the water enable it to conduct. Water Conduct Electricity Physics.
From pemandangan-keren.blogspot.com
Which Is The Most Good Conductor Of Electricity Nice Pic Water Conduct Electricity Physics But provide an ionic compound in the form of salt, and you complete the circuit. There are no ions in pure water, like fully deionized or distilled water. The conductivity of water is a measure of the capability of water to pass electrical flow. The conductivity of water is a measure of the capability of water to pass electrical flow.. Water Conduct Electricity Physics.
From edu.dfrobot.com
Can pure water conduct electricity? DFRobot Science Lab EP10 DFRobot Water Conduct Electricity Physics There are no ions in pure water, like fully deionized or distilled water. This ability directly depends on the concentration of conductive. Pure water is an electrical insulator. Any impurities, like salts, in the water enable it to conduct electricity. The conductivity of water is a measure of the capability of water to pass electrical flow. Pure water doesn’t conduct. Water Conduct Electricity Physics.
From www.dreamstime.com
Electricity Compared To Water in Labeled Educational Physics Outline Water Conduct Electricity Physics When salts are dissolved in water, they. Learn the factors affecting water conductivity and its importance here The conductivity of water is a measure of the capability of water to pass electrical flow. Water’s ability to conduct electricity hinges on the presence of ion—charged particles. Any impurities, like salts, in the water enable it to conduct electricity. The conductivity of. Water Conduct Electricity Physics.
From www.thoughtco.com
10 Examples of Electrical Conductors and Insulators Water Conduct Electricity Physics Water’s ability to conduct electricity hinges on the presence of ion—charged particles. Pure water is a very poor conductor (resistivity is actually used as a measure of purity). Learn the factors affecting water conductivity and its importance here When salts are dissolved in water, they. Pure water doesn’t conduct electricity. There are no ions in pure water, like fully deionized. Water Conduct Electricity Physics.