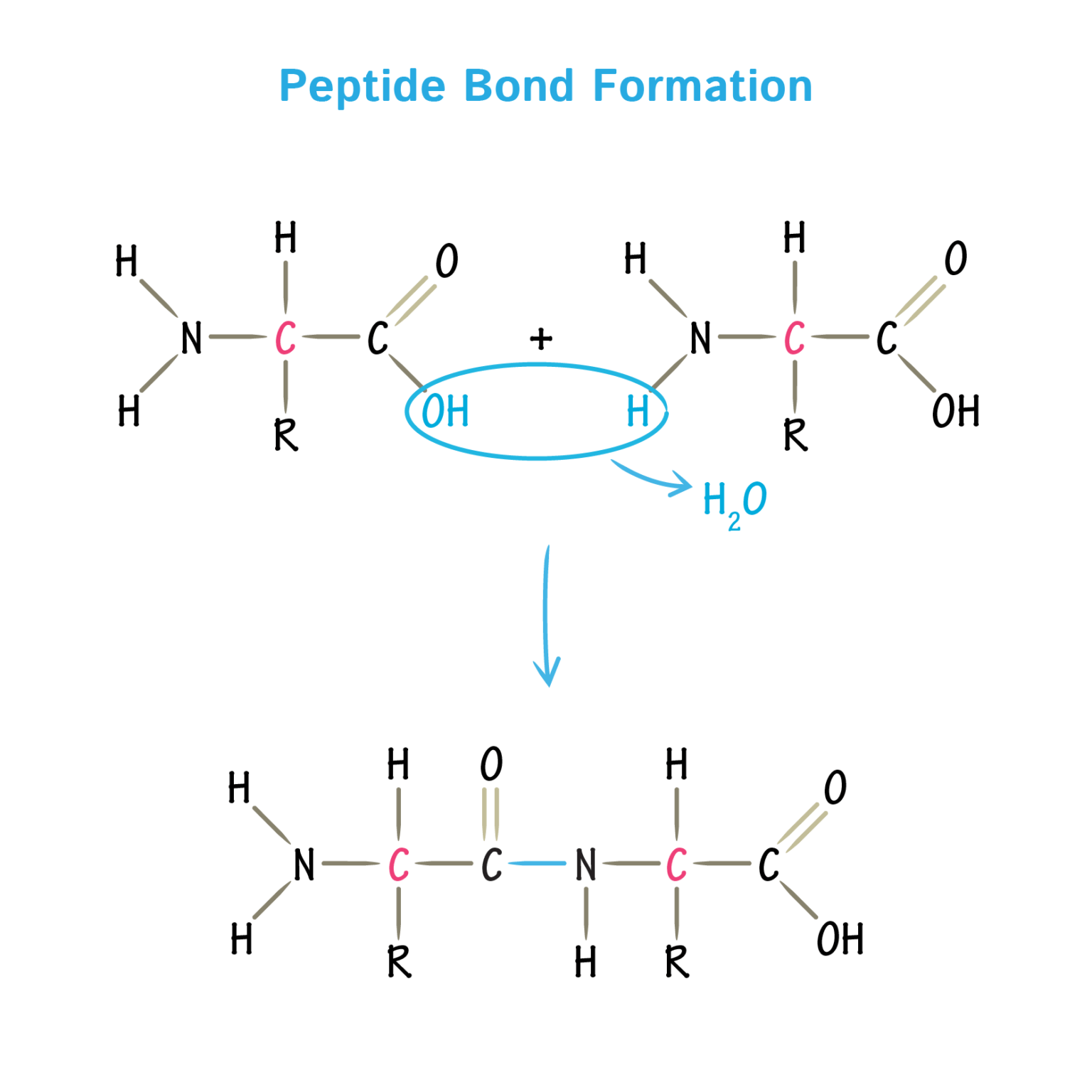
What Bonds Hold Monomers Together . Step reactions and chain reactions. Put them together to form one part, and they describe a monomer: Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. When two amino acids are covalently attached. There are two types of polymerization reactions: One common natural monomer is. Two separate molecules bind together, forming a covalent bond. Any one molecule that joins with other monomers to create a larger molecule. The individual proteins in the assembly are called monomers or subunits. Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such.
from microbiologynotes.org
Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. Put them together to form one part, and they describe a monomer: Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. One common natural monomer is. The individual proteins in the assembly are called monomers or subunits. There are two types of polymerization reactions: Two separate molecules bind together, forming a covalent bond. Any one molecule that joins with other monomers to create a larger molecule. The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. Step reactions and chain reactions.
Amino acids physical, chemical properties and peptide bond
What Bonds Hold Monomers Together Step reactions and chain reactions. Any one molecule that joins with other monomers to create a larger molecule. The individual proteins in the assembly are called monomers or subunits. When two amino acids are covalently attached. Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. Two separate molecules bind together, forming a covalent bond. There are two types of polymerization reactions: Step reactions and chain reactions. Put them together to form one part, and they describe a monomer: The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. One common natural monomer is.
From quizlet.com
BIO 1A What is a cell? Monomers and Polymers. Diagram Quizlet What Bonds Hold Monomers Together Any one molecule that joins with other monomers to create a larger molecule. The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. Step reactions and chain reactions. The individual proteins in the assembly are called monomers or subunits. There are two types of polymerization reactions: One common natural monomer is. Two. What Bonds Hold Monomers Together.
From byjus.com
The reaction where a phosphate groups bond to a nucleotides to form a What Bonds Hold Monomers Together Step reactions and chain reactions. There are two types of polymerization reactions: Any one molecule that joins with other monomers to create a larger molecule. Put them together to form one part, and they describe a monomer: The individual proteins in the assembly are called monomers or subunits. The submit are held together by the same interactions that hold the. What Bonds Hold Monomers Together.
From slideplayer.com
Chapter 2 Biochemistry. ppt download What Bonds Hold Monomers Together There are two types of polymerization reactions: When two amino acids are covalently attached. Put them together to form one part, and they describe a monomer: One common natural monomer is. Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. The submit are held together by the same interactions that hold. What Bonds Hold Monomers Together.
From www.toppr.com
Bond Linking Monomers Types with Concepts, Videos and Examples What Bonds Hold Monomers Together There are two types of polymerization reactions: Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. Two separate molecules bind together, forming a covalent bond. Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. When two. What Bonds Hold Monomers Together.
From pediaa.com
How are Proteins Constructed from Amino Acids Monomers of Proteins What Bonds Hold Monomers Together There are two types of polymerization reactions: Two separate molecules bind together, forming a covalent bond. Any one molecule that joins with other monomers to create a larger molecule. Step reactions and chain reactions. Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. When two amino acids are covalently attached. Polymers. What Bonds Hold Monomers Together.
From www.numerade.com
SOLVED What type of molecules act as enzymes? nin What is the monomer What Bonds Hold Monomers Together The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. Two separate molecules bind together, forming a covalent bond. When two amino acids are covalently attached. One common natural monomer is. There are two types of polymerization reactions: Step reactions and chain reactions. The individual proteins in the assembly are called monomers. What Bonds Hold Monomers Together.
From sciencetrends.com
What Are The Monomers Of Lipids? Science Trends What Bonds Hold Monomers Together One common natural monomer is. Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. Two separate molecules bind together, forming a covalent bond. When two amino acids are covalently attached. Step reactions and chain reactions. Put them together to form one part, and they describe. What Bonds Hold Monomers Together.
From www.youtube.com
Bonding, monomers and polymers AQA A Level Biology YouTube What Bonds Hold Monomers Together One common natural monomer is. The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. There are two types of polymerization reactions: Any one molecule that joins with other monomers to create a larger molecule. When two amino acids are covalently attached. Two separate molecules bind together, forming a covalent bond. The. What Bonds Hold Monomers Together.
From www.linstitute.net
AQA A Level Biology复习笔记1.1.5 The Glycosidic Bond翰林国际教育 What Bonds Hold Monomers Together Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. Step reactions and chain reactions. The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. When two amino acids are covalently attached. Put them together to form one. What Bonds Hold Monomers Together.
From microbiologynotes.org
Amino acids physical, chemical properties and peptide bond What Bonds Hold Monomers Together Any one molecule that joins with other monomers to create a larger molecule. Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. The individual proteins in the. What Bonds Hold Monomers Together.
From www.chegg.com
Solved What types of bond hold together monomers within a What Bonds Hold Monomers Together Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. Step reactions and chain reactions. Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. The individual proteins in the assembly are called monomers or subunits. Put them. What Bonds Hold Monomers Together.
From selena-kcardenas.blogspot.com
What Is the General Term for Any Carbohydrate Monomer What Bonds Hold Monomers Together Put them together to form one part, and they describe a monomer: When two amino acids are covalently attached. The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. One common natural monomer is. Step reactions and chain reactions. Polymers can form via a condensation reaction, in which two monomer molecules are. What Bonds Hold Monomers Together.
From www.numerade.com
SOLVED In DNA, where do the bonds occur that hold the nucleotide What Bonds Hold Monomers Together Put them together to form one part, and they describe a monomer: Two separate molecules bind together, forming a covalent bond. The individual proteins in the assembly are called monomers or subunits. Any one molecule that joins with other monomers to create a larger molecule. There are two types of polymerization reactions: Polymers can form via a condensation reaction, in. What Bonds Hold Monomers Together.
From www.slideserve.com
PPT New Section Nucleic Acids final group of macromolecules What Bonds Hold Monomers Together There are two types of polymerization reactions: Any one molecule that joins with other monomers to create a larger molecule. One common natural monomer is. The individual proteins in the assembly are called monomers or subunits. Two separate molecules bind together, forming a covalent bond. Polymers can form via a condensation reaction, in which two monomer molecules are joined by. What Bonds Hold Monomers Together.
From www.numerade.com
SOLVED Starch is made up of glucose monomers. Not all starch molecules What Bonds Hold Monomers Together The individual proteins in the assembly are called monomers or subunits. Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. When two amino acids are covalently attached. Two separate molecules bind together, forming a covalent bond. Any one molecule that joins with other monomers to. What Bonds Hold Monomers Together.
From www.naxlex.com
The covalent bonds between the monomers of an enzyme... What Bonds Hold Monomers Together One common natural monomer is. Put them together to form one part, and they describe a monomer: The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. Any. What Bonds Hold Monomers Together.
From www.toppr.com
Bond Linking Monomers Types with Concepts, Videos and Examples What Bonds Hold Monomers Together Put them together to form one part, and they describe a monomer: When two amino acids are covalently attached. The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. The individual proteins in the assembly are called monomers or subunits. One common natural monomer is. Two separate molecules bind together, forming a. What Bonds Hold Monomers Together.
From www.slideserve.com
PPT Monomers and Polymers PowerPoint Presentation ID2116472 What Bonds Hold Monomers Together Step reactions and chain reactions. Any one molecule that joins with other monomers to create a larger molecule. When two amino acids are covalently attached. Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. Put them together to form one part, and they describe a. What Bonds Hold Monomers Together.
From www.expii.com
How Do Macromolecules Form? — Overview & Process Expii What Bonds Hold Monomers Together Step reactions and chain reactions. Two separate molecules bind together, forming a covalent bond. The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. Put them together to form one part, and they describe a monomer: One common natural monomer is. Each amino acid is attached to another amino acid by a. What Bonds Hold Monomers Together.
From www.chemistrylearner.com
Chemical Bonds Definition, Types, and Examples What Bonds Hold Monomers Together Step reactions and chain reactions. Any one molecule that joins with other monomers to create a larger molecule. Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. Two separate molecules bind together, forming a covalent bond. One common natural monomer is. The individual proteins in. What Bonds Hold Monomers Together.
From www.alamy.com
Scientific Designing of Glycosidic Bonds. Glycosidic Bond Formation What Bonds Hold Monomers Together One common natural monomer is. The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. Two separate molecules bind together, forming a covalent bond. The individual proteins in the assembly are called monomers or subunits. There are two types of polymerization reactions: When two amino acids are covalently attached. Each amino acid. What Bonds Hold Monomers Together.
From www.chegg.com
Solved The monomers of macromolecules are held together What Bonds Hold Monomers Together Put them together to form one part, and they describe a monomer: Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. Any one molecule that joins with other monomers to create a larger molecule. The submit are held together by the same interactions that hold. What Bonds Hold Monomers Together.
From www.alamy.com
Propylene (propene) and polypropylene (PP, polypropene) molecule What Bonds Hold Monomers Together Two separate molecules bind together, forming a covalent bond. Put them together to form one part, and they describe a monomer: Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. Each amino acid is attached to another amino acid by a covalent bond, known as. What Bonds Hold Monomers Together.
From www.slideserve.com
PPT Monomers and Polymers PowerPoint Presentation, free download ID What Bonds Hold Monomers Together There are two types of polymerization reactions: Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. The individual proteins in the assembly are called monomers or subunits. Step reactions and chain reactions. When two amino acids are covalently attached. One common natural monomer is. Put them together to form one part,. What Bonds Hold Monomers Together.
From www.slideserve.com
PPT New Section Nucleic Acids final group of macromolecules What Bonds Hold Monomers Together Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. When two amino acids are covalently attached. Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. Any one molecule that joins with other monomers to create a. What Bonds Hold Monomers Together.
From www.numerade.com
SOLVED Match the type of macromolecule with the type of bond used to What Bonds Hold Monomers Together When two amino acids are covalently attached. Put them together to form one part, and they describe a monomer: Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. Two separate molecules bind together, forming a covalent bond. One common natural monomer is. Any one molecule. What Bonds Hold Monomers Together.
From quizdbbarnstorms.z21.web.core.windows.net
What Bonds Hold Together Dna What Bonds Hold Monomers Together Any one molecule that joins with other monomers to create a larger molecule. Two separate molecules bind together, forming a covalent bond. One common natural monomer is. There are two types of polymerization reactions: Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. The submit. What Bonds Hold Monomers Together.
From www.slideserve.com
PPT The process of joining together monomers is known as What Bonds Hold Monomers Together The individual proteins in the assembly are called monomers or subunits. Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. Two separate molecules bind together, forming a covalent bond. The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. Step reactions and chain. What Bonds Hold Monomers Together.
From studylib.net
Chemical bonds hold molecules together atoms formed when they indicate the What Bonds Hold Monomers Together Step reactions and chain reactions. Any one molecule that joins with other monomers to create a larger molecule. When two amino acids are covalently attached. Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. There are two types of polymerization reactions: Put them together to form one part, and they describe. What Bonds Hold Monomers Together.
From www.biologyonline.com
Monomer Definition and Examples Biology Online Dictionary What Bonds Hold Monomers Together The individual proteins in the assembly are called monomers or subunits. The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. There are two types of polymerization reactions: When two amino acids are covalently attached. Any one molecule that joins with other monomers to create a larger molecule. Step reactions and chain. What Bonds Hold Monomers Together.
From socratic.org
What are the monomers of the nucleic acid polymers deoxyribonucleic What Bonds Hold Monomers Together When two amino acids are covalently attached. Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. The individual proteins in the assembly are called monomers or subunits.. What Bonds Hold Monomers Together.
From brainly.com
Glucose molecules bond together in a process known as dehydration What Bonds Hold Monomers Together Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. Put them together to form one part, and they describe a monomer: Any one molecule that joins with other monomers to create a larger molecule. When two amino acids are covalently attached. One common natural monomer is. Two separate molecules bind together,. What Bonds Hold Monomers Together.
From www.numerade.com
SOLVED During the process below, specific pairing occurs between the What Bonds Hold Monomers Together Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. Put them together to form one part, and they describe a monomer: One common natural monomer is. Two. What Bonds Hold Monomers Together.
From www.slideserve.com
PPT Monomers and Polymers PowerPoint Presentation ID2116472 What Bonds Hold Monomers Together The submit are held together by the same interactions that hold the tertiary structure of individual subunits, i.e.,. Step reactions and chain reactions. Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. When two amino acids are covalently attached. One common natural monomer is. Polymers can form via a condensation reaction,. What Bonds Hold Monomers Together.
From present5.com
What do you know about these scientists What Bonds Hold Monomers Together The individual proteins in the assembly are called monomers or subunits. There are two types of polymerization reactions: Put them together to form one part, and they describe a monomer: Polymers can form via a condensation reaction, in which two monomer molecules are joined by a new covalent bond and a small molecule such. Any one molecule that joins with. What Bonds Hold Monomers Together.