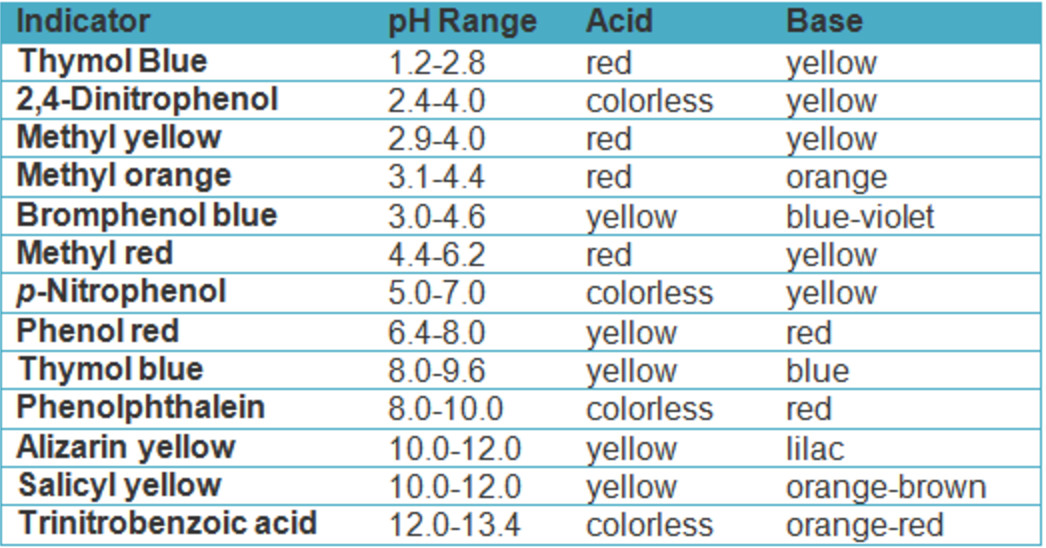
Indicator For Strong Base Titration . As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. (a) red prior to the end point; Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. This page assumes that you know about ph curves for all the. Methyl red’s endpoint for the titration of a strong acid with a strong base; Select an appropriate indicator for a given. Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. An aqueous solution of ammonia, nh 3 (aq), is a.
from classnotes.org.in
As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. This page assumes that you know about ph curves for all the. Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. Select an appropriate indicator for a given. Methyl red’s endpoint for the titration of a strong acid with a strong base; An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. (a) red prior to the end point; An aqueous solution of ammonia, nh 3 (aq), is a.
Acid Base Titration using Indicator Chemistry, Class 11, Ionic
Indicator For Strong Base Titration Methyl red’s endpoint for the titration of a strong acid with a strong base; Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. This page assumes that you know about ph curves for all the. Methyl red’s endpoint for the titration of a strong acid with a strong base; An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. An aqueous solution of ammonia, nh 3 (aq), is a. (a) red prior to the end point; Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. Select an appropriate indicator for a given.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Indicator For Strong Base Titration An aqueous solution of ammonia, nh 3 (aq), is a. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. Select an appropriate indicator for a given. This page assumes that you know about ph curves for all the. Titration of a strong acid with a strong base is the simplest of the four types of titrations as. Indicator For Strong Base Titration.
From mmerevise.co.uk
pH Curves Questions and Revision MME Indicator For Strong Base Titration (a) red prior to the end point; This page assumes that you know about ph curves for all the. Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid.. Indicator For Strong Base Titration.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Indicator For Strong Base Titration (a) red prior to the end point; Select an appropriate indicator for a given. Methyl red’s endpoint for the titration of a strong acid with a strong base; Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. An aqueous solution of hydrochloric acid,. Indicator For Strong Base Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Indicator For Strong Base Titration Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. Select an appropriate indicator for a given. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. An aqueous solution of ammonia, nh 3 (aq), is a. This page. Indicator For Strong Base Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicator For Strong Base Titration Methyl red’s endpoint for the titration of a strong acid with a strong base; An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. This page assumes that you. Indicator For Strong Base Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicator For Strong Base Titration An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. (a) red prior to the end point; Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used. Indicator For Strong Base Titration.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Indicator For Strong Base Titration An aqueous solution of ammonia, nh 3 (aq), is a. This page assumes that you know about ph curves for all the. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong.. Indicator For Strong Base Titration.
From www.hotzxgirl.com
Solved Choose The Correct Indicator For Acid Base Titration Chegg Hot Indicator For Strong Base Titration Methyl red’s endpoint for the titration of a strong acid with a strong base; Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. Select. Indicator For Strong Base Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator For Strong Base Titration An aqueous solution of ammonia, nh 3 (aq), is a. Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. Select an appropriate indicator for a given. Methyl red’s. Indicator For Strong Base Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicator For Strong Base Titration As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. Titration of a. Indicator For Strong Base Titration.
From www.slideshare.net
Acid base titration Indicator For Strong Base Titration (a) red prior to the end point; Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. Select an appropriate indicator for a given. Titration of a strong acid. Indicator For Strong Base Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download Indicator For Strong Base Titration As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. This page assumes that you know about ph curves for all the. An aqueous solution of hydrochloric acid,. Indicator For Strong Base Titration.
From www.numerade.com
SOLVED bromothymol blue indicator is not a good indicator for strong Indicator For Strong Base Titration Select an appropriate indicator for a given. Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. (a) red prior to the end point; An aqueous solution of ammonia, nh 3 (aq), is a. This page assumes that you know about. Indicator For Strong Base Titration.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID Indicator For Strong Base Titration An aqueous solution of ammonia, nh 3 (aq), is a. Select an appropriate indicator for a given. (a) red prior to the end point; This page assumes that you know about ph curves for all the. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. Methyl red’s endpoint for the titration of a. Indicator For Strong Base Titration.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Indicator For Strong Base Titration Select an appropriate indicator for a given. (a) red prior to the end point; Methyl red’s endpoint for the titration of a strong acid with a strong base; As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. Determine ph and concentrations of chemical species at any point in the titration of a strong. Indicator For Strong Base Titration.
From sciencenotes.org
Phenolphthalein Indicator Indicator For Strong Base Titration An aqueous solution of ammonia, nh 3 (aq), is a. (a) red prior to the end point; Methyl red’s endpoint for the titration of a strong acid with a strong base; Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid.. Indicator For Strong Base Titration.
From mungfali.com
Acid Base Titration Indicator Indicator For Strong Base Titration Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. An aqueous solution of ammonia, nh 3 (aq), is a. (a) red prior to the end point; As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. Indicator For Strong Base Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicator For Strong Base Titration Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. This page assumes that you know about ph curves for all the. Select an appropriate indicator for a given. Methyl red’s endpoint for the titration of a strong acid with a strong base; As. Indicator For Strong Base Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Indicator For Strong Base Titration As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. Methyl red’s endpoint for the titration of a strong acid with a strong base; Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. This page assumes that. Indicator For Strong Base Titration.
From mungfali.com
Acid Base Titration Indicator Indicator For Strong Base Titration Methyl red’s endpoint for the titration of a strong acid with a strong base; Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong. Indicator For Strong Base Titration.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Indicator For Strong Base Titration Select an appropriate indicator for a given. This page assumes that you know about ph curves for all the. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. Titration of a strong acid with a strong base is the simplest of. Indicator For Strong Base Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicator For Strong Base Titration Methyl red’s endpoint for the titration of a strong acid with a strong base; Select an appropriate indicator for a given. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. This page assumes that you know about ph curves for all the. An aqueous solution of ammonia, nh 3 (aq), is a. Titration. Indicator For Strong Base Titration.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Indicator For Strong Base Titration An aqueous solution of ammonia, nh 3 (aq), is a. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. Methyl red’s endpoint for the titration of a strong acid with a strong base; Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid. Indicator For Strong Base Titration.
From www.sliderbase.com
Titration Indicator For Strong Base Titration An aqueous solution of ammonia, nh 3 (aq), is a. (a) red prior to the end point; This page assumes that you know about ph curves for all the. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. Titration of a. Indicator For Strong Base Titration.
From mungfali.com
Acid Base Titration Procedure Indicator For Strong Base Titration Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. (a) red prior to the end point; Select an appropriate indicator for a given. This page assumes that you know about ph curves for all the. As seen in the chapter. Indicator For Strong Base Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicator For Strong Base Titration As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. (a) red prior to the end point; Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. An aqueous solution of hydrochloric acid, hcl (aq),. Indicator For Strong Base Titration.
From ar.inspiredpencil.com
Titration Diagram Indicator For Strong Base Titration Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. An aqueous solution of hydrochloric. Indicator For Strong Base Titration.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle Indicator For Strong Base Titration As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. Titration of a strong acid with a strong base is the simplest of the four. Indicator For Strong Base Titration.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Indicator For Strong Base Titration This page assumes that you know about ph curves for all the. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. Methyl red’s endpoint for the titration of a strong acid with a strong base; Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a. Indicator For Strong Base Titration.
From hxedcejuo.blob.core.windows.net
Titration Indicator Strong Base at Patricia Walker blog Indicator For Strong Base Titration As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. An aqueous solution of ammonia, nh 3 (aq), is a. This page assumes that you. Indicator For Strong Base Titration.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Indicator For Strong Base Titration Methyl red’s endpoint for the titration of a strong acid with a strong base; This page assumes that you know about ph curves for all the. Select an appropriate indicator for a given. An aqueous solution of ammonia, nh 3 (aq), is a. (a) red prior to the end point; Determine ph and concentrations of chemical species at any point. Indicator For Strong Base Titration.
From alexus-bogspotcortez.blogspot.com
How to Know Which Indicator to Use for Titration Indicator For Strong Base Titration An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. Determine ph and concentrations of chemical species at any point in the titration of a strong acid by a strong base or a strong base by a strong acid. (a) red prior to the end point; Titration of a strong acid with a strong base is the simplest. Indicator For Strong Base Titration.
From www.chegg.com
Solved 1. Figure 1 shows the titration curves of four Indicator For Strong Base Titration Methyl red’s endpoint for the titration of a strong acid with a strong base; Select an appropriate indicator for a given. Titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. (a) red prior to the end point; An aqueous solution of hydrochloric acid,. Indicator For Strong Base Titration.
From ecampusontario.pressbooks.pub
6.3 AcidBase Reactions & Titrations General Chemistry for GeeGees Indicator For Strong Base Titration This page assumes that you know about ph curves for all the. Methyl red’s endpoint for the titration of a strong acid with a strong base; As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. An aqueous solution of ammonia, nh 3 (aq), is a. An aqueous solution of hydrochloric acid, hcl (aq),. Indicator For Strong Base Titration.
From mungfali.com
Weak Acid Vs Strong Base Titration Curve Indicator For Strong Base Titration As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to. (a) red prior to the end point; An aqueous solution of ammonia, nh 3 (aq), is a. Select an appropriate indicator for a given. Methyl red’s endpoint for the titration of a strong acid with a strong base; An aqueous solution of hydrochloric acid,. Indicator For Strong Base Titration.