Sodium Hydroxide Used In Experiment . learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear. Much like a spy, the. sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. The solution is then standardized, that is, its concentration is. 10 analysis of acids and bases by titration. in the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Molar mass = 204.2 g/mol) as an analyte, and sodium. in this experiment you will use the titration technique to determine the concentration of the provided sodium hydroxide solution. The process of carrying out a. in this experiment, you will use potassium hydrogen phthalate (, khp;
from www.laboratorynotes.com
in this experiment you will use the titration technique to determine the concentration of the provided sodium hydroxide solution. 10 analysis of acids and bases by titration. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. in the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. The process of carrying out a. Much like a spy, the. Molar mass = 204.2 g/mol) as an analyte, and sodium. sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. The solution is then standardized, that is, its concentration is. learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear.
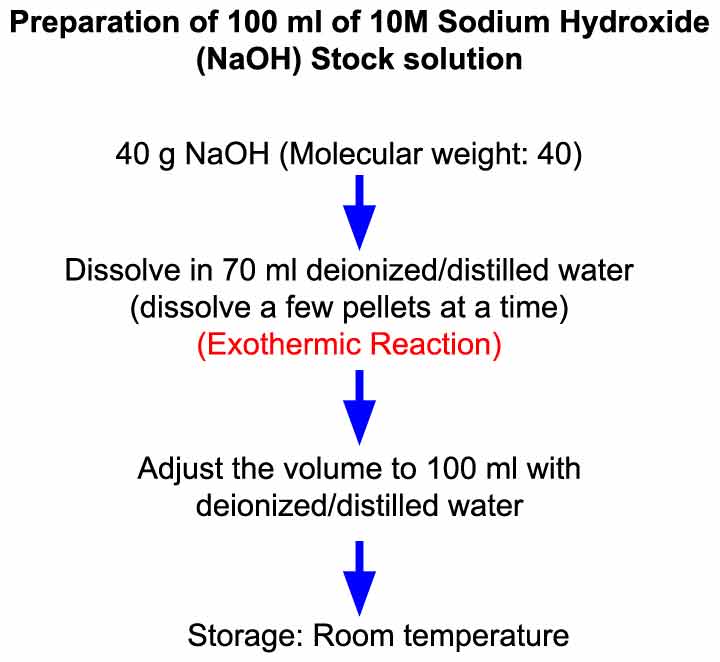
Preparation of 10 M Sodium Hydroxide (NaOH) Solution Laboratory Notes
Sodium Hydroxide Used In Experiment Molar mass = 204.2 g/mol) as an analyte, and sodium. in this experiment you will use the titration technique to determine the concentration of the provided sodium hydroxide solution. Molar mass = 204.2 g/mol) as an analyte, and sodium. The solution is then standardized, that is, its concentration is. in this experiment, you will use potassium hydrogen phthalate (, khp; learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear. in the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. The process of carrying out a. Much like a spy, the. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. 10 analysis of acids and bases by titration.
From www.pinterest.ph
Sodium hydroxide vector illustration VectorMine Chemistry lessons Sodium Hydroxide Used In Experiment The process of carrying out a. in this experiment, you will use potassium hydrogen phthalate (, khp; in the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Molar mass = 204.2 g/mol) as an analyte, and sodium. learn about acid/base reactions as sodium hydroxide reacts with gas in the. Sodium Hydroxide Used In Experiment.
From mungfali.com
Structure Of Sodium Hydroxide Sodium Hydroxide Used In Experiment in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. 10 analysis of acids and bases by titration. The process of carrying out a. The solution is then standardized, that is, its concentration is. learn about acid/base reactions as sodium hydroxide reacts with gas in the air to. Sodium Hydroxide Used In Experiment.
From www.myxxgirl.com
Neutralisation Experiment Hydrochloric Acid And Sodium Hydroxide My Sodium Hydroxide Used In Experiment The solution is then standardized, that is, its concentration is. sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. 10 analysis of acids and bases by titration. Much like a spy, the. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride. Sodium Hydroxide Used In Experiment.
From www.pinterest.com
Pin on Lifehacker & Experimenter Sodium Hydroxide Used In Experiment in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The process of carrying out a. in this experiment, you will use potassium hydrogen phthalate (, khp; sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. 10 analysis of. Sodium Hydroxide Used In Experiment.
From www.youtube.com
How to prepare and standardize 0.1 N Sodium Hydroxide(NaOH) Solution Sodium Hydroxide Used In Experiment Molar mass = 204.2 g/mol) as an analyte, and sodium. The process of carrying out a. in this experiment, you will use potassium hydrogen phthalate (, khp; learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear. in this experiment students neutralise sodium hydroxide with hydrochloric. Sodium Hydroxide Used In Experiment.
From www.thoughtco.com
Where to Buy Sodium Hydroxide Sodium Hydroxide Used In Experiment in the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. in this experiment, you will use potassium hydrogen phthalate (, khp; Much like a spy, the. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. in this experiment. Sodium Hydroxide Used In Experiment.
From www.sciencephoto.com
Sodium hydroxide Stock Image A710/0035 Science Photo Library Sodium Hydroxide Used In Experiment Much like a spy, the. in this experiment you will use the titration technique to determine the concentration of the provided sodium hydroxide solution. in the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. The solution is then standardized, that is, its concentration is. sodium hydroxide solution of about. Sodium Hydroxide Used In Experiment.
From www.slideserve.com
PPT Sodium Hydroxide Experiment With Electrolysis PowerPoint Sodium Hydroxide Used In Experiment in this experiment, you will use potassium hydrogen phthalate (, khp; learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear. The process of carrying out a. in the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Much. Sodium Hydroxide Used In Experiment.
From www.laboratorynotes.com
Preparation of 10 M Sodium Hydroxide (NaOH) Solution Laboratory Notes Sodium Hydroxide Used In Experiment The solution is then standardized, that is, its concentration is. learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear. in this experiment you will use the titration technique to determine the concentration of the provided sodium hydroxide solution. Molar mass = 204.2 g/mol) as an analyte,. Sodium Hydroxide Used In Experiment.
From edurev.in
What are the uses of Sodium Hydroxide (NaOH)? EduRev Class 10 Question Sodium Hydroxide Used In Experiment Molar mass = 204.2 g/mol) as an analyte, and sodium. Much like a spy, the. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The process of carrying out a. 10 analysis of acids and bases by titration. in the neutralization of hydrochloric acid by sodium hydroxide,. Sodium Hydroxide Used In Experiment.
From www.studocu.com
Experiment 3 lab report Experiment 3 PREPARATION OF A STANDARD Sodium Hydroxide Used In Experiment The solution is then standardized, that is, its concentration is. learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear. The process of carrying out a. Much like a spy, the. 10 analysis of acids and bases by titration. in the neutralization of hydrochloric acid by. Sodium Hydroxide Used In Experiment.
From melscience.com
Sodium hydroxide, solution 1 M MEL Chemistry Sodium Hydroxide Used In Experiment Much like a spy, the. in this experiment, you will use potassium hydrogen phthalate (, khp; sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. 10 analysis of acids and bases by titration. The solution is then standardized, that is, its concentration is. in the neutralization of hydrochloric. Sodium Hydroxide Used In Experiment.
From www.dreamstime.com
Illustration, Sodium Hydroxide in Glass, Chemical in the Laboratory and Sodium Hydroxide Used In Experiment in this experiment you will use the titration technique to determine the concentration of the provided sodium hydroxide solution. in this experiment, you will use potassium hydrogen phthalate (, khp; Much like a spy, the. Molar mass = 204.2 g/mol) as an analyte, and sodium. sodium hydroxide solution of about 0.2 m is prepared in order to. Sodium Hydroxide Used In Experiment.
From www.youtube.com
Acetic acid (ethanoic acid) and Sodium hydroxide reaction CH3COOH Sodium Hydroxide Used In Experiment in this experiment, you will use potassium hydrogen phthalate (, khp; The process of carrying out a. Much like a spy, the. The solution is then standardized, that is, its concentration is. Molar mass = 204.2 g/mol) as an analyte, and sodium. sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp. Sodium Hydroxide Used In Experiment.
From studylib.net
Experiment (1) Standardization of sodium hydroxide NaOH solution Sodium Hydroxide Used In Experiment sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. Much like a spy, the. learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear. in this experiment, you will use potassium hydrogen phthalate (, khp; The solution is. Sodium Hydroxide Used In Experiment.
From dokumen.tips
(PDF) Chemistry 102 EXPERIMENT 9B Sodium Hydroxide Used In Experiment The solution is then standardized, that is, its concentration is. in this experiment, you will use potassium hydrogen phthalate (, khp; sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. in the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. . Sodium Hydroxide Used In Experiment.
From cartoondealer.com
Sodium Hydroxide In Bottle , Chemical In The Laboratory And Industry Sodium Hydroxide Used In Experiment sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. Molar mass = 204.2 g/mol) as an analyte, and sodium. in the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. in this experiment, you will use potassium hydrogen phthalate (, khp; . Sodium Hydroxide Used In Experiment.
From www.alamy.com
Molecular Model of Sodium Hydroxide (NaOH) Molecule. Vector Sodium Hydroxide Used In Experiment learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear. 10 analysis of acids and bases by titration. in this experiment, you will use potassium hydrogen phthalate (, khp; The process of carrying out a. in this experiment you will use the titration technique to. Sodium Hydroxide Used In Experiment.
From www.diycraftcorner.com
The Role of Sodium Hydroxide in Soap Making DIY Craft Corner Sodium Hydroxide Used In Experiment in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Much like a spy, the. in this experiment, you will use potassium hydrogen phthalate (, khp; Molar mass = 204.2 g/mol) as an analyte, and sodium. learn about acid/base reactions as sodium hydroxide reacts with gas in the. Sodium Hydroxide Used In Experiment.
From plagiarismonline.web.fc2.com
Analysis of acid by titration with sodium hydroxide Sodium Hydroxide Used In Experiment in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Much like a spy, the. in this experiment, you will use potassium hydrogen phthalate (, khp; learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear. Molar. Sodium Hydroxide Used In Experiment.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Sodium Hydroxide Used In Experiment The process of carrying out a. Molar mass = 204.2 g/mol) as an analyte, and sodium. in the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Much like a spy, the. in this experiment you will use the titration technique to determine the concentration of the provided sodium hydroxide solution.. Sodium Hydroxide Used In Experiment.
From www.youtube.com
What happens when Sodium Hydroxide and Aluminium Reacts Sodium Hydroxide Used In Experiment sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. Molar mass = 204.2 g/mol) as an analyte, and sodium. in this experiment you will use the titration technique to determine the concentration of the provided sodium hydroxide solution. 10 analysis of acids and bases by titration. in this. Sodium Hydroxide Used In Experiment.
From www.youtube.com
Preparation & Standardization of 0.1N Sodium Hydroxide (NaOH) Solution Sodium Hydroxide Used In Experiment Much like a spy, the. in this experiment, you will use potassium hydrogen phthalate (, khp; 10 analysis of acids and bases by titration. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. in the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of. Sodium Hydroxide Used In Experiment.
From mireyakruwhamilton.blogspot.com
Sodium Hydroxide Molar Mass MireyakruwHamilton Sodium Hydroxide Used In Experiment in this experiment, you will use potassium hydrogen phthalate (, khp; The solution is then standardized, that is, its concentration is. in the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. Molar mass = 204.2 g/mol) as an analyte, and sodium. learn about acid/base reactions as sodium hydroxide reacts. Sodium Hydroxide Used In Experiment.
From evekruwhoffman.blogspot.com
Standardizing a Solution of Sodium Hydroxide EvekruwHoffman Sodium Hydroxide Used In Experiment in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The process of carrying out a. sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce. Sodium Hydroxide Used In Experiment.
From insende.netlify.app
33++ Hydrochloric Acid And Sodium Hydroxide Reaction Insende Sodium Hydroxide Used In Experiment in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. 10 analysis of acids and bases by titration. The solution is then standardized, that is, its concentration is. Molar mass = 204.2 g/mol) as an analyte, and sodium. sodium hydroxide solution of about 0.2 m is prepared in. Sodium Hydroxide Used In Experiment.
From teachernotes4u.com
Diagram shows the apparatus setup to study the reaction between sodium Sodium Hydroxide Used In Experiment Molar mass = 204.2 g/mol) as an analyte, and sodium. in the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The process of carrying out a. learn about acid/base reactions. Sodium Hydroxide Used In Experiment.
From www.sjzxlwhg.com
Properties of Sodium Hydroxide And Notes on Its Use Sodium Hydroxide Used In Experiment The solution is then standardized, that is, its concentration is. in this experiment, you will use potassium hydrogen phthalate (, khp; The process of carrying out a. learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear. sodium hydroxide solution of about 0.2 m is prepared. Sodium Hydroxide Used In Experiment.
From www.youtube.com
Sodium Hydroxide and Aluminum Experiment YouTube Sodium Hydroxide Used In Experiment Molar mass = 204.2 g/mol) as an analyte, and sodium. The solution is then standardized, that is, its concentration is. in this experiment you will use the titration technique to determine the concentration of the provided sodium hydroxide solution. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution.. Sodium Hydroxide Used In Experiment.
From webapi.bu.edu
Standardizing a solution of sodium hydroxide lab report answers. Lab Sodium Hydroxide Used In Experiment in this experiment you will use the titration technique to determine the concentration of the provided sodium hydroxide solution. Molar mass = 204.2 g/mol) as an analyte, and sodium. sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. in this experiment students neutralise sodium hydroxide with hydrochloric acid to. Sodium Hydroxide Used In Experiment.
From www.dreamstime.com
Illustration, Sodium Hydroxide in Glass, Chemical in the Laboratory and Sodium Hydroxide Used In Experiment in this experiment, you will use potassium hydrogen phthalate (, khp; in the neutralization of hydrochloric acid by sodium hydroxide, the mole ratio of acid to base is 1:1. in this experiment you will use the titration technique to determine the concentration of the provided sodium hydroxide solution. The solution is then standardized, that is, its concentration. Sodium Hydroxide Used In Experiment.
From www.realclearscience.com
Aluminum + Sodium Hydroxide Produces a Mess and a Boom RealClearScience Sodium Hydroxide Used In Experiment learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear. The process of carrying out a. Molar mass = 204.2 g/mol) as an analyte, and sodium. Much like a spy, the. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium. Sodium Hydroxide Used In Experiment.
From www.thoughtco.com
How to Prepare a Sodium Hydroxide or NaOH Solution Sodium Hydroxide Used In Experiment learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear. 10 analysis of acids and bases by titration. Molar mass = 204.2 g/mol) as an analyte, and sodium. in this experiment you will use the titration technique to determine the concentration of the provided sodium hydroxide. Sodium Hydroxide Used In Experiment.
From www.youtube.com
Producing NaOH (Sodium Hydroxide) experiment YouTube Sodium Hydroxide Used In Experiment The process of carrying out a. in this experiment, you will use potassium hydrogen phthalate (, khp; Much like a spy, the. learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear. in this experiment you will use the titration technique to determine the concentration of. Sodium Hydroxide Used In Experiment.
From studylib.net
Standardizing a Sodium Hydroxide (NaOH) Solution Sodium Hydroxide Used In Experiment Much like a spy, the. The solution is then standardized, that is, its concentration is. The process of carrying out a. learn about acid/base reactions as sodium hydroxide reacts with gas in the air to produce a solution that appears to disappear. 10 analysis of acids and bases by titration. in this experiment, you will use potassium. Sodium Hydroxide Used In Experiment.