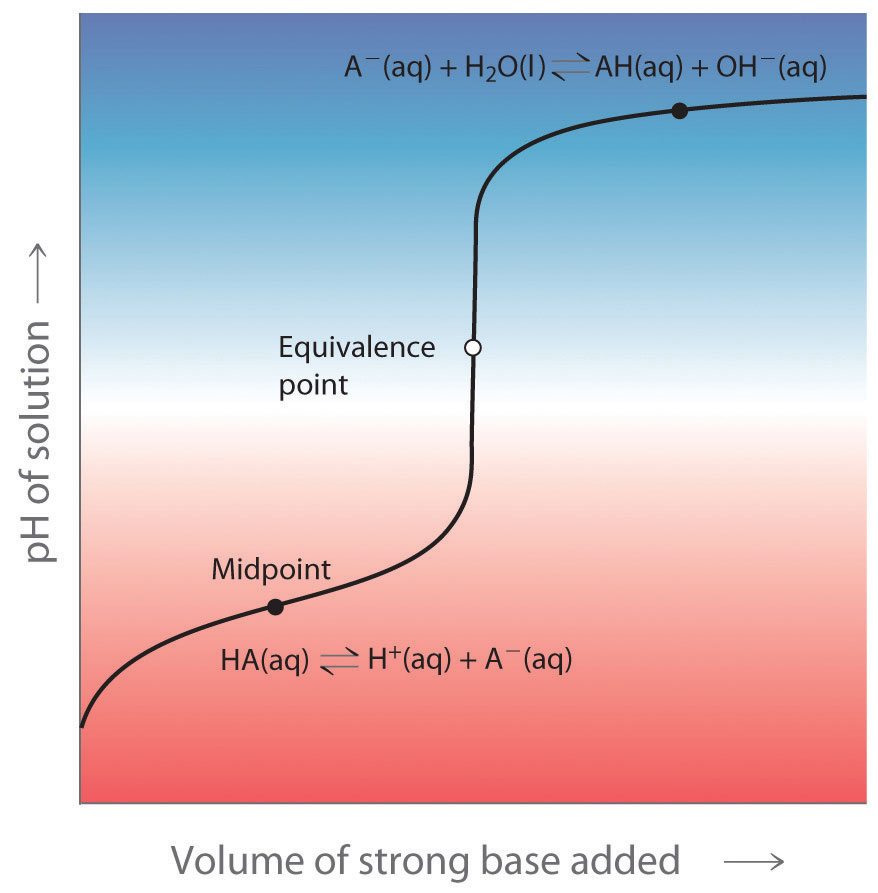
What Does A Buffer Do In Chemistry . in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. Selecting proper components for desired ph. what is a buffer composed of? buffers do so by being composed of certain pairs of solutes: what is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of. Either a weak acid plus a salt derived from that weak acid or a weak. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It consists of a solution of a weak acid. How does a buffer work?
from chem.libretexts.org
How does a buffer work? in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. what is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of. It consists of a solution of a weak acid. what is a buffer composed of? a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Selecting proper components for desired ph. buffers do so by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid or a weak.
Chapter 16.6 Buffers Chemistry LibreTexts
What Does A Buffer Do In Chemistry It consists of a solution of a weak acid. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Selecting proper components for desired ph. Either a weak acid plus a salt derived from that weak acid or a weak. It consists of a solution of a weak acid. How does a buffer work? buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. what is a buffer composed of? in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A solution whose ph is not altered to any great extent by the addition of small quantities of. buffers do so by being composed of certain pairs of solutes: what is buffer in chemistry?
From www.brainkart.com
How do buffers work? What Does A Buffer Do In Chemistry It consists of a solution of a weak acid. buffers do so by being composed of certain pairs of solutes: what is a buffer composed of? Selecting proper components for desired ph. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. buffer, in. What Does A Buffer Do In Chemistry.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Does A Buffer Do In Chemistry Either a weak acid plus a salt derived from that weak acid or a weak. buffers do so by being composed of certain pairs of solutes: a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. How does a buffer work? what is buffer in. What Does A Buffer Do In Chemistry.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide What Does A Buffer Do In Chemistry It consists of a solution of a weak acid. How does a buffer work? what is buffer in chemistry? Either a weak acid plus a salt derived from that weak acid or a weak. A solution whose ph is not altered to any great extent by the addition of small quantities of. buffers do so by being composed. What Does A Buffer Do In Chemistry.
From courses.lumenlearning.com
Buffers Chemistry for Majors What Does A Buffer Do In Chemistry a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Either a weak acid plus a salt derived from that weak acid. What Does A Buffer Do In Chemistry.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium What Does A Buffer Do In Chemistry what is buffer in chemistry? in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. A solution whose ph is not. What Does A Buffer Do In Chemistry.
From www.pinterest.co.uk
Buffer Easy Science Chemistry education, Study chemistry, Chemistry classroom What Does A Buffer Do In Chemistry a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. what is a buffer composed of? what is buffer in chemistry? buffers do so by being composed of certain pairs of solutes: A solution whose ph is not altered to any great extent by. What Does A Buffer Do In Chemistry.
From www.slideshare.net
Buffer system What Does A Buffer Do In Chemistry what is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of. buffers do so by being composed of certain pairs of solutes: what is a buffer composed of? Either a weak acid plus a salt derived from that weak acid or a weak. buffer,. What Does A Buffer Do In Chemistry.
From www.youtube.com
How Do Buffers Work? With Buffer Calculations! AP Chemistry Unit 8.3 YouTube What Does A Buffer Do In Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of. buffers do so by being composed of certain pairs of solutes: a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. How does a buffer work? Selecting proper. What Does A Buffer Do In Chemistry.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Does A Buffer Do In Chemistry what is buffer in chemistry? in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. How does a buffer work? It consists of a solution of a weak acid. A solution whose ph is not altered to any great extent by the addition of. What Does A Buffer Do In Chemistry.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Does A Buffer Do In Chemistry It consists of a solution of a weak acid. A solution whose ph is not altered to any great extent by the addition of small quantities of. How does a buffer work? a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Either a weak acid plus. What Does A Buffer Do In Chemistry.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Does A Buffer Do In Chemistry buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. what is a buffer composed of? what is buffer in. What Does A Buffer Do In Chemistry.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download ID5348308 What Does A Buffer Do In Chemistry buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. Selecting proper components for desired ph. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A solution whose ph is not altered to. What Does A Buffer Do In Chemistry.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation What Does A Buffer Do In Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of. what is a buffer composed of? Either a weak acid plus a salt derived from that weak acid or a weak. It consists of a solution of a weak acid. what is buffer in chemistry? buffer, in chemistry, solution. What Does A Buffer Do In Chemistry.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs What Does A Buffer Do In Chemistry Either a weak acid plus a salt derived from that weak acid or a weak. It consists of a solution of a weak acid. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. buffers do so by being composed of certain pairs of solutes: . What Does A Buffer Do In Chemistry.
From www.peoi.org
Chapter 12 Section G Buffers What Does A Buffer Do In Chemistry what is a buffer composed of? A solution whose ph is not altered to any great extent by the addition of small quantities of. in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. How does a buffer work? buffers do so by. What Does A Buffer Do In Chemistry.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Does A Buffer Do In Chemistry Selecting proper components for desired ph. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. How does a buffer work? in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A. What Does A Buffer Do In Chemistry.
From www.slideserve.com
PPT All About Buffers PowerPoint Presentation, free download ID1321295 What Does A Buffer Do In Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of. what is buffer in chemistry? It consists of a solution of a weak acid. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Selecting proper components for. What Does A Buffer Do In Chemistry.
From magmastory.blogspot.com
Which Of The Following Represents A Buffer System? magmastory What Does A Buffer Do In Chemistry Either a weak acid plus a salt derived from that weak acid or a weak. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. How does a buffer work? what is buffer in chemistry? what is a buffer composed of? Selecting proper components for. What Does A Buffer Do In Chemistry.
From www.youtube.com
Buffer capacity Acids and bases AP Chemistry Khan Academy YouTube What Does A Buffer Do In Chemistry How does a buffer work? buffers do so by being composed of certain pairs of solutes: buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. what is buffer in chemistry? a buffer is a solution that maintains the stability of a system’s ph. What Does A Buffer Do In Chemistry.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Does A Buffer Do In Chemistry How does a buffer work? what is buffer in chemistry? what is a buffer composed of? buffers do so by being composed of certain pairs of solutes: Selecting proper components for desired ph. It consists of a solution of a weak acid. Either a weak acid plus a salt derived from that weak acid or a weak.. What Does A Buffer Do In Chemistry.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Presentation ID2529504 What Does A Buffer Do In Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of. what is buffer in chemistry? buffers do so by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid or a weak. How does a buffer work? buffer, in chemistry,. What Does A Buffer Do In Chemistry.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Does A Buffer Do In Chemistry Selecting proper components for desired ph. what is a buffer composed of? a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Either a weak acid plus a salt derived from that weak acid or a weak. what is buffer in chemistry? How does a. What Does A Buffer Do In Chemistry.
From thechemistrynotes.com
Buffer Solution Types, Properties, and Uses What Does A Buffer Do In Chemistry It consists of a solution of a weak acid. A solution whose ph is not altered to any great extent by the addition of small quantities of. Either a weak acid plus a salt derived from that weak acid or a weak. buffers do so by being composed of certain pairs of solutes: a buffer is a solution. What Does A Buffer Do In Chemistry.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its action. YouTube What Does A Buffer Do In Chemistry what is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of. in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. buffers do so by being composed of certain pairs of. What Does A Buffer Do In Chemistry.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Does A Buffer Do In Chemistry Selecting proper components for desired ph. what is a buffer composed of? buffers do so by being composed of certain pairs of solutes: what is buffer in chemistry? Either a weak acid plus a salt derived from that weak acid or a weak. A solution whose ph is not altered to any great extent by the addition. What Does A Buffer Do In Chemistry.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 What Does A Buffer Do In Chemistry buffers do so by being composed of certain pairs of solutes: Selecting proper components for desired ph. How does a buffer work? what is a buffer composed of? in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Either a weak acid plus. What Does A Buffer Do In Chemistry.
From chem.libretexts.org
Chapter 16.6 Buffers Chemistry LibreTexts What Does A Buffer Do In Chemistry buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. Either a weak acid plus a salt derived from that weak acid or a weak. A solution whose ph is not altered to any great extent by the addition of small quantities of. what is buffer. What Does A Buffer Do In Chemistry.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Experiment GoldBio What Does A Buffer Do In Chemistry buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Either a weak acid plus a salt derived from that weak acid or a. What Does A Buffer Do In Chemistry.
From www.youtube.com
How do buffers work, paper 1 AQA A level Chemistry YouTube What Does A Buffer Do In Chemistry what is a buffer composed of? in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. buffers do so by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid or a weak. A. What Does A Buffer Do In Chemistry.
From www.thoughtco.com
What Is a Buffer and How Does It Work? What Does A Buffer Do In Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of. How does a buffer work? buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. buffers do so by being composed of certain pairs of solutes: It consists. What Does A Buffer Do In Chemistry.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Experiment GoldBio What Does A Buffer Do In Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of. How does a buffer work? in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. a buffer is a solution that maintains the stability of a. What Does A Buffer Do In Chemistry.
From www.slideserve.com
PPT Buffers and Salts PowerPoint Presentation, free download ID5405242 What Does A Buffer Do In Chemistry what is a buffer composed of? Selecting proper components for desired ph. Either a weak acid plus a salt derived from that weak acid or a weak. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. what is buffer in chemistry? A solution whose. What Does A Buffer Do In Chemistry.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Does A Buffer Do In Chemistry what is a buffer composed of? It consists of a solution of a weak acid. Selecting proper components for desired ph. Either a weak acid plus a salt derived from that weak acid or a weak. buffers do so by being composed of certain pairs of solutes: A solution whose ph is not altered to any great extent. What Does A Buffer Do In Chemistry.
From mydigitalkemistry.com
What is a buffer solution simple definition? Free Chemistry Learning Platform What Does A Buffer Do In Chemistry It consists of a solution of a weak acid. A solution whose ph is not altered to any great extent by the addition of small quantities of. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. buffers do so by being composed of certain pairs. What Does A Buffer Do In Chemistry.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation What Does A Buffer Do In Chemistry It consists of a solution of a weak acid. what is buffer in chemistry? buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. in chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid. What Does A Buffer Do In Chemistry.