Copper Oxide Empirical Formula Experiment . What is the empirical formula of copper oxide based on your results in experiment 1? What is an empirical formula for copper (ii) oxide? What color was the copper (ii). In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. The empirical formula of a copper oxide reading assignment: Why magnesium or calcium cannot replace copper oxide? The copper(ii) oxide is heated strongly using a bunsen burner; You calculate the molar ratios of each element in the oxide. Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed; Measure the mass of the tube. What is the empirical formula of copper oxide based on your results in experiment 1? Chang, chemistry 10th edition, pp. When a 2.50 g sample of copper is heated, it forms 3.13 g of.
from www.numerade.com
The copper(ii) oxide is heated strongly using a bunsen burner; Measure the mass of the tube. You calculate the molar ratios of each element in the oxide. In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. The empirical formula of a copper oxide reading assignment: Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed; What is the empirical formula of copper oxide based on your results in experiment 1? What is the empirical formula of copper oxide based on your results in experiment 1? What is an empirical formula for copper (ii) oxide? When a 2.50 g sample of copper is heated, it forms 3.13 g of.
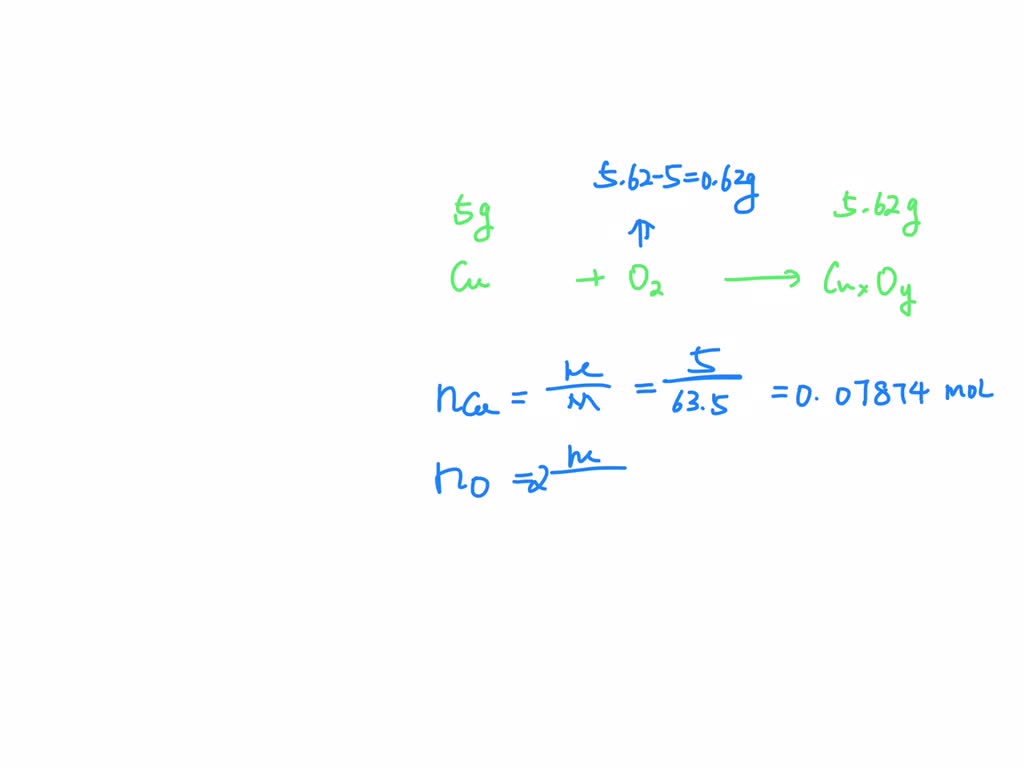
SOLVED If 5.00 grams of copper metal reacts with oxygen to produce 5.62 grams of 'copper oxide
Copper Oxide Empirical Formula Experiment When a 2.50 g sample of copper is heated, it forms 3.13 g of. Measure the mass of the tube. What is the empirical formula of copper oxide based on your results in experiment 1? The empirical formula of a copper oxide reading assignment: What color was the copper (ii). Chang, chemistry 10th edition, pp. Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed; You calculate the molar ratios of each element in the oxide. The copper(ii) oxide is heated strongly using a bunsen burner; What is the empirical formula of copper oxide based on your results in experiment 1? What is an empirical formula for copper (ii) oxide? In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. Why magnesium or calcium cannot replace copper oxide? When a 2.50 g sample of copper is heated, it forms 3.13 g of.
From www.youtube.com
form 4 chem chap 3 empirical formula experiment for CuO YouTube Copper Oxide Empirical Formula Experiment Chang, chemistry 10th edition, pp. You calculate the molar ratios of each element in the oxide. What is an empirical formula for copper (ii) oxide? The copper(ii) oxide is heated strongly using a bunsen burner; In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and. Copper Oxide Empirical Formula Experiment.
From www.chegg.com
1 Empirical Formula of Copper Oxide_Essay1_Mass after Copper Oxide Empirical Formula Experiment Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed; What is the empirical formula of copper oxide based on your results in experiment 1? In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. Measure the mass. Copper Oxide Empirical Formula Experiment.
From www.studypool.com
SOLUTION The empirical formula of a copper oxide Studypool Copper Oxide Empirical Formula Experiment Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed; What is the empirical formula of copper oxide based on your results in experiment 1? What is an empirical formula for copper (ii) oxide? When a 2.50 g sample of copper is heated, it forms 3.13 g of. What color was the copper (ii). Chang,. Copper Oxide Empirical Formula Experiment.
From studylib.net
Determination of the Empirical Formula of Copper Oxide Copper Oxide Empirical Formula Experiment What is an empirical formula for copper (ii) oxide? What is the empirical formula of copper oxide based on your results in experiment 1? What color was the copper (ii). When a 2.50 g sample of copper is heated, it forms 3.13 g of. Measure the mass of the tube. You calculate the molar ratios of each element in the. Copper Oxide Empirical Formula Experiment.
From www.studypool.com
SOLUTION Determination of the empirical formula of copper oxide Studypool Copper Oxide Empirical Formula Experiment You calculate the molar ratios of each element in the oxide. The empirical formula of a copper oxide reading assignment: Chang, chemistry 10th edition, pp. The copper(ii) oxide is heated strongly using a bunsen burner; What color was the copper (ii). Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed; What is an empirical. Copper Oxide Empirical Formula Experiment.
From atelier-yuwa.ciao.jp
Finding The Formula Of Copper(II) Oxide Experiment RSC Education atelieryuwa.ciao.jp Copper Oxide Empirical Formula Experiment Measure the mass of the tube. Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed; Why magnesium or calcium cannot replace copper oxide? The empirical formula of a copper oxide reading assignment: Chang, chemistry 10th edition, pp. What color was the copper (ii). The copper(ii) oxide is heated strongly using a bunsen burner; What. Copper Oxide Empirical Formula Experiment.
From studylib.net
Empirical formula of copper oxidestudent Copper Oxide Empirical Formula Experiment What is the empirical formula of copper oxide based on your results in experiment 1? The copper(ii) oxide is heated strongly using a bunsen burner; What is the empirical formula of copper oxide based on your results in experiment 1? Why magnesium or calcium cannot replace copper oxide? You calculate the molar ratios of each element in the oxide. Chang,. Copper Oxide Empirical Formula Experiment.
From www.studypool.com
SOLUTION The empirical formula of a copper oxide Studypool Copper Oxide Empirical Formula Experiment In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. Measure the mass of the tube. When a 2.50 g sample of copper is heated, it forms 3.13 g of. The empirical formula of a copper oxide reading assignment: Heat until metal oxide. Copper Oxide Empirical Formula Experiment.
From www.studypool.com
SOLUTION Notes on determination of the empirical formula of copper oxide (empirical formula of Copper Oxide Empirical Formula Experiment What is the empirical formula of copper oxide based on your results in experiment 1? Why magnesium or calcium cannot replace copper oxide? In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. Heat until metal oxide completely changes colour, meaning that all. Copper Oxide Empirical Formula Experiment.
From studylib.net
Determination of the Empirical Formula of Copper Oxide Introduction Copper Oxide Empirical Formula Experiment Chang, chemistry 10th edition, pp. The empirical formula of a copper oxide reading assignment: What is the empirical formula of copper oxide based on your results in experiment 1? Why magnesium or calcium cannot replace copper oxide? You calculate the molar ratios of each element in the oxide. The copper(ii) oxide is heated strongly using a bunsen burner; What color. Copper Oxide Empirical Formula Experiment.
From studylib.net
90 Finding the formula of an oxide of copper Copper Oxide Empirical Formula Experiment In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. Chang, chemistry 10th edition, pp. What color was the copper (ii). Measure the mass of the tube. The copper(ii) oxide is heated strongly using a bunsen burner; The empirical formula of a copper. Copper Oxide Empirical Formula Experiment.
From www.youtube.com
Experiment The Empirical Formula of a Copper Oxide. Part 2/8 The parts of the Flame YouTube Copper Oxide Empirical Formula Experiment When a 2.50 g sample of copper is heated, it forms 3.13 g of. The empirical formula of a copper oxide reading assignment: In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. Heat until metal oxide completely changes colour, meaning that all. Copper Oxide Empirical Formula Experiment.
From hxejfqksb.blob.core.windows.net
Copper Oxide Empirical Formula Lab at Theron Dobson blog Copper Oxide Empirical Formula Experiment Why magnesium or calcium cannot replace copper oxide? Chang, chemistry 10th edition, pp. The copper(ii) oxide is heated strongly using a bunsen burner; The empirical formula of a copper oxide reading assignment: What is an empirical formula for copper (ii) oxide? What is the empirical formula of copper oxide based on your results in experiment 1? Measure the mass of. Copper Oxide Empirical Formula Experiment.
From www.chegg.com
Solved Question 17 Consider the reaction of copper (II) Copper Oxide Empirical Formula Experiment What is an empirical formula for copper (ii) oxide? You calculate the molar ratios of each element in the oxide. Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed; What is the empirical formula of copper oxide based on your results in experiment 1? Why magnesium or calcium cannot replace copper oxide? The empirical. Copper Oxide Empirical Formula Experiment.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:1.5.7 Practical Determine the Formula of a Metal Oxide Copper Oxide Empirical Formula Experiment Chang, chemistry 10th edition, pp. In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. What is an empirical formula for copper (ii) oxide? You calculate the molar ratios of each element in the oxide. The empirical formula of a copper oxide reading. Copper Oxide Empirical Formula Experiment.
From www.studypool.com
SOLUTION Discovering the Empirical Formula of Copper Oxide A HandsOn Lab Experiment Studypool Copper Oxide Empirical Formula Experiment The copper(ii) oxide is heated strongly using a bunsen burner; When a 2.50 g sample of copper is heated, it forms 3.13 g of. Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed; Measure the mass of the tube. What is the empirical formula of copper oxide based on your results in experiment 1?. Copper Oxide Empirical Formula Experiment.
From www.youtube.com
Lab Lecture Empirical Formula of Copper Oxide YouTube Copper Oxide Empirical Formula Experiment Why magnesium or calcium cannot replace copper oxide? What is the empirical formula of copper oxide based on your results in experiment 1? Chang, chemistry 10th edition, pp. The copper(ii) oxide is heated strongly using a bunsen burner; In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy,. Copper Oxide Empirical Formula Experiment.
From www.studocu.com
Experimental Chemistry Emperical Formula Copper Oxide. Lab 3 Empirical Formula Copper Oxide Copper Oxide Empirical Formula Experiment What is the empirical formula of copper oxide based on your results in experiment 1? Why magnesium or calcium cannot replace copper oxide? Chang, chemistry 10th edition, pp. In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. What is an empirical formula. Copper Oxide Empirical Formula Experiment.
From www.studocu.com
Empirical formula of copper oxide 1 Empirical Formula of Copper Oxide_Essay1_Mass after Copper Oxide Empirical Formula Experiment When a 2.50 g sample of copper is heated, it forms 3.13 g of. What is the empirical formula of copper oxide based on your results in experiment 1? Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed; The empirical formula of a copper oxide reading assignment: You calculate the molar ratios of each. Copper Oxide Empirical Formula Experiment.
From keplarllp.com
🎉 Determination of the empirical formula of copper oxide. How can I calculate the empirical Copper Oxide Empirical Formula Experiment What color was the copper (ii). You calculate the molar ratios of each element in the oxide. The copper(ii) oxide is heated strongly using a bunsen burner; The empirical formula of a copper oxide reading assignment: What is the empirical formula of copper oxide based on your results in experiment 1? Heat until metal oxide completely changes colour, meaning that. Copper Oxide Empirical Formula Experiment.
From studylib.net
Determination of the Empirical Formula of Copper Oxide Copper Oxide Empirical Formula Experiment The copper(ii) oxide is heated strongly using a bunsen burner; In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. What color was the copper (ii). What is the empirical formula of copper oxide based on your results in experiment 1? Heat until. Copper Oxide Empirical Formula Experiment.
From www.chegg.com
1 Empirical Formula of Copper Oxide_Essay1_Mass after Copper Oxide Empirical Formula Experiment What is the empirical formula of copper oxide based on your results in experiment 1? Why magnesium or calcium cannot replace copper oxide? Chang, chemistry 10th edition, pp. The copper(ii) oxide is heated strongly using a bunsen burner; Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed; In this experiment you will be given. Copper Oxide Empirical Formula Experiment.
From www.numerade.com
SOLVED If 5.00 grams of copper metal reacts with oxygen to produce 5.62 grams of 'copper oxide Copper Oxide Empirical Formula Experiment Measure the mass of the tube. Chang, chemistry 10th edition, pp. When a 2.50 g sample of copper is heated, it forms 3.13 g of. In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. What is an empirical formula for copper (ii). Copper Oxide Empirical Formula Experiment.
From www.studypool.com
SOLUTION Notes on determination of the empirical formula of copper oxide (empirical formula of Copper Oxide Empirical Formula Experiment The empirical formula of a copper oxide reading assignment: In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. You calculate the molar ratios of each element in the oxide. What is an empirical formula for copper (ii) oxide? When a 2.50 g. Copper Oxide Empirical Formula Experiment.
From www.studypool.com
SOLUTION The empirical formula of a copper oxide Studypool Copper Oxide Empirical Formula Experiment When a 2.50 g sample of copper is heated, it forms 3.13 g of. What color was the copper (ii). Measure the mass of the tube. The empirical formula of a copper oxide reading assignment: What is an empirical formula for copper (ii) oxide? In this experiment you will be given a sample of copper oxide and your task is. Copper Oxide Empirical Formula Experiment.
From www.slideshare.net
Short Chem Exercises Copper Oxide Empirical Formula Experiment The copper(ii) oxide is heated strongly using a bunsen burner; Why magnesium or calcium cannot replace copper oxide? What is the empirical formula of copper oxide based on your results in experiment 1? Measure the mass of the tube. Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed; In this experiment you will be. Copper Oxide Empirical Formula Experiment.
From www.youtube.com
Experiment The Empirical Formula of a Copper Oxide. Part 6/8 YouTube Copper Oxide Empirical Formula Experiment What color was the copper (ii). You calculate the molar ratios of each element in the oxide. The copper(ii) oxide is heated strongly using a bunsen burner; Measure the mass of the tube. When a 2.50 g sample of copper is heated, it forms 3.13 g of. Chang, chemistry 10th edition, pp. What is an empirical formula for copper (ii). Copper Oxide Empirical Formula Experiment.
From www.studypool.com
SOLUTION Notes on determination of the empirical formula of copper oxide (empirical formula of Copper Oxide Empirical Formula Experiment Measure the mass of the tube. In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. The empirical formula of a copper oxide reading assignment: What is the empirical formula of copper oxide based on your results in experiment 1? What color was. Copper Oxide Empirical Formula Experiment.
From www.studypool.com
SOLUTION Determination of Empirical Formula (Copper Oxide) Lab Studypool Copper Oxide Empirical Formula Experiment What color was the copper (ii). What is an empirical formula for copper (ii) oxide? Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed; You calculate the molar ratios of each element in the oxide. Chang, chemistry 10th edition, pp. What is the empirical formula of copper oxide based on your results in experiment. Copper Oxide Empirical Formula Experiment.
From resource.studiaacademy.com
Chemical Formulae, Equations and Calculations Studia Academy Resources Copper Oxide Empirical Formula Experiment Chang, chemistry 10th edition, pp. The empirical formula of a copper oxide reading assignment: What is an empirical formula for copper (ii) oxide? Why magnesium or calcium cannot replace copper oxide? In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. Heat until. Copper Oxide Empirical Formula Experiment.
From www.youtube.com
Finding the empirical formula of copper oxide YouTube Copper Oxide Empirical Formula Experiment What color was the copper (ii). What is the empirical formula of copper oxide based on your results in experiment 1? What is the empirical formula of copper oxide based on your results in experiment 1? Measure the mass of the tube. Chang, chemistry 10th edition, pp. Heat until metal oxide completely changes colour, meaning that all the oxygen has. Copper Oxide Empirical Formula Experiment.
From www.studypool.com
SOLUTION Determination of the empirical formula of copper oxide Studypool Copper Oxide Empirical Formula Experiment What is the empirical formula of copper oxide based on your results in experiment 1? What color was the copper (ii). In this experiment you will be given a sample of copper oxide and your task is to determine its empirical formula, cuxoy, where x and y are. You calculate the molar ratios of each element in the oxide. The. Copper Oxide Empirical Formula Experiment.
From www.chegg.com
Solved HelpEmpirical Formula Of Copper Oxide Experiment Copper Oxide Empirical Formula Experiment Why magnesium or calcium cannot replace copper oxide? Chang, chemistry 10th edition, pp. When a 2.50 g sample of copper is heated, it forms 3.13 g of. You calculate the molar ratios of each element in the oxide. What color was the copper (ii). Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed; Measure. Copper Oxide Empirical Formula Experiment.
From www.studocu.com
Empirical Formula of Copper Oxide Adimaio Empirical Formula of Copper Oxide Alyssa DiMaio Copper Oxide Empirical Formula Experiment What is the empirical formula of copper oxide based on your results in experiment 1? Why magnesium or calcium cannot replace copper oxide? What is the empirical formula of copper oxide based on your results in experiment 1? The empirical formula of a copper oxide reading assignment: What color was the copper (ii). Measure the mass of the tube. Heat. Copper Oxide Empirical Formula Experiment.
From www.youtube.com
Experiment The Empirical Formula of a Copper Oxide. Part 5/8 YouTube Copper Oxide Empirical Formula Experiment You calculate the molar ratios of each element in the oxide. When a 2.50 g sample of copper is heated, it forms 3.13 g of. What is the empirical formula of copper oxide based on your results in experiment 1? What color was the copper (ii). The copper(ii) oxide is heated strongly using a bunsen burner; What is an empirical. Copper Oxide Empirical Formula Experiment.