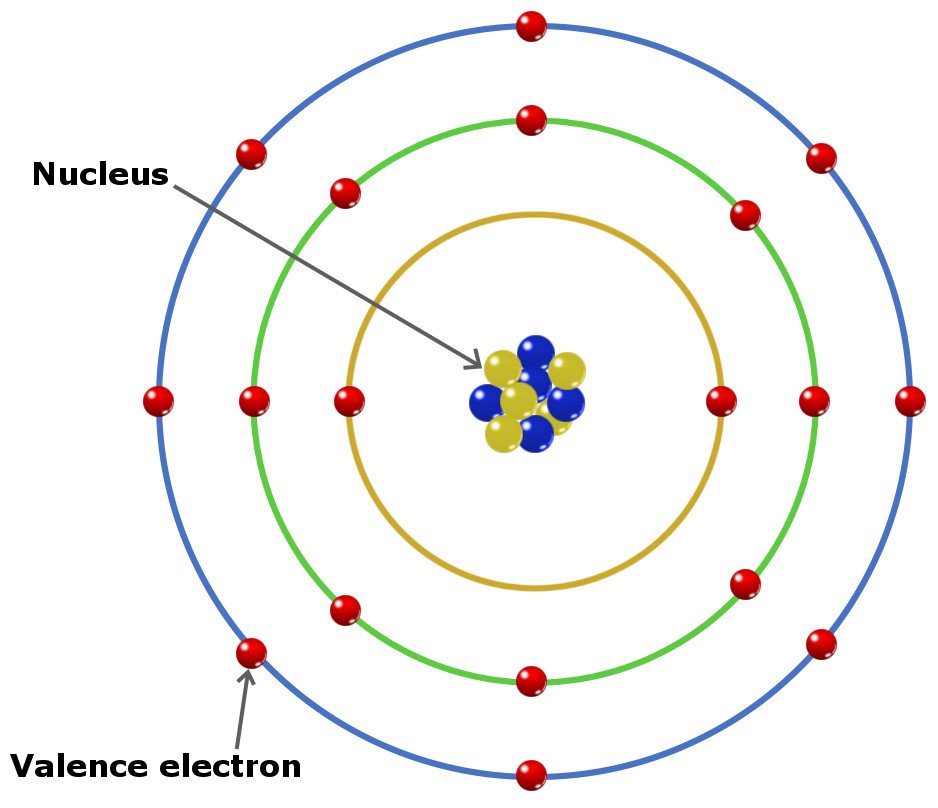
How Do You Work Out How Many Electrons An Element Has . The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. For example, identify the element of an atom with 54. The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. The electronic structure of an atom can be predicted from its atomic number. No matter how many electrons or neutrons an atom has, the element is defined by its. Describe the locations, charges, and masses of the three main subatomic particles. Each element is defined by the number of protons found in each of its atoms. Determine the number of protons and electrons. Therefore, you can determine the number of protons if the number of electrons is given. Finding the number of electrons. Number of protons = number of electrons.
from www.scienceabc.com
Number of protons = number of electrons. Each element is defined by the number of protons found in each of its atoms. The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Determine the number of protons and electrons. Therefore, you can determine the number of protons if the number of electrons is given. Finding the number of electrons. No matter how many electrons or neutrons an atom has, the element is defined by its. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. Describe the locations, charges, and masses of the three main subatomic particles.
What Are Valence Electrons And How To Find Them? Where Are They Located?
How Do You Work Out How Many Electrons An Element Has The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. Finding the number of electrons. No matter how many electrons or neutrons an atom has, the element is defined by its. Number of protons = number of electrons. Determine the number of protons and electrons. Each element is defined by the number of protons found in each of its atoms. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Therefore, you can determine the number of protons if the number of electrons is given. The electronic structure of an atom can be predicted from its atomic number. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. Describe the locations, charges, and masses of the three main subatomic particles. For example, identify the element of an atom with 54. The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after.
From topblogtenz.com
How to find Valence electrons Various method and Examples How Do You Work Out How Many Electrons An Element Has Each element is defined by the number of protons found in each of its atoms. Number of protons = number of electrons. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. Determine the number of protons and electrons. No matter how many electrons or neutrons an atom has, the element. How Do You Work Out How Many Electrons An Element Has.
From www.periodictableprintable.com
How Do You Find Protons Neutrons And Electrons On The Periodic Table How Do You Work Out How Many Electrons An Element Has The electronic structure of an atom can be predicted from its atomic number. The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. Determine the number of protons and electrons. Therefore, you can determine the number of protons if the number of electrons is given. Finding the number of electrons. The number. How Do You Work Out How Many Electrons An Element Has.
From periodictable.me
FindtheNumberofNeutronsinanAtomStep11preview.jpg Dynamic How Do You Work Out How Many Electrons An Element Has For example, identify the element of an atom with 54. Each element is defined by the number of protons found in each of its atoms. Describe the locations, charges, and masses of the three main subatomic particles. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. Finding the number of. How Do You Work Out How Many Electrons An Element Has.
From utedzz.blogspot.com
Periodic Table Of Elements List With Protons Neutrons And Electrons How Do You Work Out How Many Electrons An Element Has Number of protons = number of electrons. The electronic structure of an atom can be predicted from its atomic number. For example, identify the element of an atom with 54. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. The number of protons is equal to the number of electrons,. How Do You Work Out How Many Electrons An Element Has.
From mk-design-group.blogspot.com
How Do You Find The Electrons Of An Element How Do You Work Out How Many Electrons An Element Has The electronic structure of an atom can be predicted from its atomic number. Finding the number of electrons. The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. Number of protons = number of electrons. For example, identify the element of an atom with 54. No matter how many electrons or neutrons. How Do You Work Out How Many Electrons An Element Has.
From www.youtube.com
Determining the number of electrons YouTube How Do You Work Out How Many Electrons An Element Has Number of protons = number of electrons. The electronic structure of an atom can be predicted from its atomic number. No matter how many electrons or neutrons an atom has, the element is defined by its. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. Each element is defined by. How Do You Work Out How Many Electrons An Element Has.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica How Do You Work Out How Many Electrons An Element Has The electronic structure of an atom can be predicted from its atomic number. Determine the number of protons and electrons. Finding the number of electrons. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. Each element is defined by the number of protons found in each of its atoms. Number. How Do You Work Out How Many Electrons An Element Has.
From sciencenotes.org
List of Electron Configurations of Elements How Do You Work Out How Many Electrons An Element Has Therefore, you can determine the number of protons if the number of electrons is given. No matter how many electrons or neutrons an atom has, the element is defined by its. Finding the number of electrons. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. Number of protons = number. How Do You Work Out How Many Electrons An Element Has.
From commons.wikimedia.org
FilePeriodic table of elements showing electron shells.png How Do You Work Out How Many Electrons An Element Has For example, identify the element of an atom with 54. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. No matter how many electrons or neutrons an atom has, the element is defined by its. Determine the number of protons and electrons. The number of protons is equal to the number of. How Do You Work Out How Many Electrons An Element Has.
From www.youtube.com
How to find the number of Protons, Neutrons and Electrons? Chemistry How Do You Work Out How Many Electrons An Element Has Determine the number of protons and electrons. Number of protons = number of electrons. Finding the number of electrons. For example, identify the element of an atom with 54. The electronic structure of an atom can be predicted from its atomic number. The number of electrons in an atom is equal to the atomic number of an element, for neutrally. How Do You Work Out How Many Electrons An Element Has.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry How Do You Work Out How Many Electrons An Element Has The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. For example, identify the element of an atom with 54. Determine the number of protons and electrons. No matter how many electrons or neutrons an atom has, the element is defined by its. Each element is defined by the number of protons. How Do You Work Out How Many Electrons An Element Has.
From www.sliderbase.com
How many protons, electrons, and neutrons are in an atom Presentation How Do You Work Out How Many Electrons An Element Has The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. The electronic structure of an atom can be predicted from its atomic number. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. The number of electrons in an atom is equal to the atomic. How Do You Work Out How Many Electrons An Element Has.
From www.scienceabc.com
What Are Valence Electrons And How To Find Them? Where Are They Located? How Do You Work Out How Many Electrons An Element Has The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. The electronic structure of an atom can be predicted from its atomic number. Therefore, you can determine the number of protons if the. How Do You Work Out How Many Electrons An Element Has.
From ppt-online.org
Properties of Atoms and the Periodic Table презентация онлайн How Do You Work Out How Many Electrons An Element Has For example, identify the element of an atom with 54. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. No matter how many electrons or neutrons an atom has, the element is defined by its. You can use these numbers to calculate the number of protons, neutrons and electrons in. How Do You Work Out How Many Electrons An Element Has.
From www.youtube.com
A Simle Guide to Finding the Numbers of Neutrons, Protons and Electrons How Do You Work Out How Many Electrons An Element Has No matter how many electrons or neutrons an atom has, the element is defined by its. The electronic structure of an atom can be predicted from its atomic number. Determine the number of protons and electrons. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Describe the locations, charges, and masses of. How Do You Work Out How Many Electrons An Element Has.
From www.youtube.com
How to Determine Number of Protons, Neutrons, and Electrons. Step by How Do You Work Out How Many Electrons An Element Has The electronic structure of an atom can be predicted from its atomic number. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. Finding the number of electrons. Therefore, you can determine the number of protons if the number of electrons is given. The number of protons is equal to the. How Do You Work Out How Many Electrons An Element Has.
From www.wikihow.com
How to Find the Number of Protons, Neutrons, and Electrons How Do You Work Out How Many Electrons An Element Has You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Each element is defined by the number of protons found in each of its atoms. The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. The number of electrons in an atom is equal to. How Do You Work Out How Many Electrons An Element Has.
From www.youtube.com
Determine the number of valence electrons from the periodic table and a How Do You Work Out How Many Electrons An Element Has No matter how many electrons or neutrons an atom has, the element is defined by its. Therefore, you can determine the number of protons if the number of electrons is given. Describe the locations, charges, and masses of the three main subatomic particles. The number of protons is equal to the number of electrons, unless there’s an ion superscript listed. How Do You Work Out How Many Electrons An Element Has.
From www.wikihow.com
How to Find Electrons 7 Steps (with Pictures) wikiHow How Do You Work Out How Many Electrons An Element Has No matter how many electrons or neutrons an atom has, the element is defined by its. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. Therefore, you can determine the number of protons if the number of electrons is given. The electronic structure of an atom can be predicted from. How Do You Work Out How Many Electrons An Element Has.
From www.wikihow.com
How to Find Electrons 6 Steps (with Pictures) wikiHow How Do You Work Out How Many Electrons An Element Has Therefore, you can determine the number of protons if the number of electrons is given. Determine the number of protons and electrons. The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. Each. How Do You Work Out How Many Electrons An Element Has.
From slidetodoc.com
Introduction to Basic Chemistry Protons Neutrons Electrons and How Do You Work Out How Many Electrons An Element Has The electronic structure of an atom can be predicted from its atomic number. Determine the number of protons and electrons. For example, identify the element of an atom with 54. The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. The number of electrons in an atom is equal to the atomic. How Do You Work Out How Many Electrons An Element Has.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy How Do You Work Out How Many Electrons An Element Has Each element is defined by the number of protons found in each of its atoms. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. Finding the number of electrons. Number of protons = number of electrons. For example, identify the element of an atom with 54. The electronic structure of. How Do You Work Out How Many Electrons An Element Has.
From www.youtube.com
How to Determine Number of Valence Electrons in Element, Ion, and How Do You Work Out How Many Electrons An Element Has Finding the number of electrons. The electronic structure of an atom can be predicted from its atomic number. Number of protons = number of electrons. Each element is defined by the number of protons found in each of its atoms. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. Determine. How Do You Work Out How Many Electrons An Element Has.
From www.wikihow.com
How to Find Electrons 6 Steps (with Pictures) wikiHow How Do You Work Out How Many Electrons An Element Has Determine the number of protons and electrons. Each element is defined by the number of protons found in each of its atoms. Therefore, you can determine the number of protons if the number of electrons is given. Finding the number of electrons. The number of electrons in an atom is equal to the atomic number of an element, for neutrally. How Do You Work Out How Many Electrons An Element Has.
From www.youtube.com
How To Calculate The Number of Protons, Neutrons, and Electrons How Do You Work Out How Many Electrons An Element Has The electronic structure of an atom can be predicted from its atomic number. No matter how many electrons or neutrons an atom has, the element is defined by its. Therefore, you can determine the number of protons if the number of electrons is given. Number of protons = number of electrons. You can use these numbers to calculate the number. How Do You Work Out How Many Electrons An Element Has.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes How Do You Work Out How Many Electrons An Element Has Finding the number of electrons. Number of protons = number of electrons. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. Determine the number of protons and electrons. Describe the locations, charges, and masses of the three main subatomic particles. For example, identify the element of an atom with 54.. How Do You Work Out How Many Electrons An Element Has.
From quizdbbarnstorms.z21.web.core.windows.net
What Element Has 8 Protons And 10 Electrons How Do You Work Out How Many Electrons An Element Has The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. Describe the locations, charges, and masses of the three main subatomic particles. The electronic structure of an atom can be predicted from its atomic number. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom.. How Do You Work Out How Many Electrons An Element Has.
From www.slideserve.com
PPT Valence Electrons PowerPoint Presentation, free download ID6712840 How Do You Work Out How Many Electrons An Element Has Each element is defined by the number of protons found in each of its atoms. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. Determine the number of protons and electrons. For example, identify the element of an atom with 54. The electronic structure of an atom can be predicted from its. How Do You Work Out How Many Electrons An Element Has.
From www.wikihow.com
How to Find Electrons 7 Steps (with Pictures) wikiHow How Do You Work Out How Many Electrons An Element Has Describe the locations, charges, and masses of the three main subatomic particles. No matter how many electrons or neutrons an atom has, the element is defined by its. For example, identify the element of an atom with 54. Determine the number of protons and electrons. Each element is defined by the number of protons found in each of its atoms.. How Do You Work Out How Many Electrons An Element Has.
From www.youtube.com
How to work out the electron configuration of an element YouTube How Do You Work Out How Many Electrons An Element Has No matter how many electrons or neutrons an atom has, the element is defined by its. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. For example, identify the element of an atom with 54. The electronic structure of an atom can be predicted from its atomic number. Number of. How Do You Work Out How Many Electrons An Element Has.
From icdsc.org
How many electrons are in each shell including 3p orbitals How Do You Work Out How Many Electrons An Element Has The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. Therefore, you can determine the number of protons if the number of electrons is given. Finding the number of electrons. You can use these numbers to calculate the number of protons, neutrons and electrons in an atom. No matter how many electrons. How Do You Work Out How Many Electrons An Element Has.
From www.wikihow.com
How to Find Electrons 7 Steps (with Pictures) wikiHow How Do You Work Out How Many Electrons An Element Has Each element is defined by the number of protons found in each of its atoms. No matter how many electrons or neutrons an atom has, the element is defined by its. The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. The electronic structure of an atom can be predicted from its. How Do You Work Out How Many Electrons An Element Has.
From quizzlibmisty.z21.web.core.windows.net
How To Find The Protons Neutrons Electrons How Do You Work Out How Many Electrons An Element Has The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. Describe the locations, charges, and masses of the three main subatomic particles. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. The electronic structure of an atom can be predicted from its. How Do You Work Out How Many Electrons An Element Has.
From www.sliderbase.com
Atoms and the Periodic table Presentation Chemistry How Do You Work Out How Many Electrons An Element Has Determine the number of protons and electrons. For example, identify the element of an atom with 54. The number of electrons in an atom is equal to the atomic number of an element, for neutrally charged. No matter how many electrons or neutrons an atom has, the element is defined by its. The electronic structure of an atom can be. How Do You Work Out How Many Electrons An Element Has.
From www.nagwa.com
Question Video Identifying the Number of Electrons in the Outermost How Do You Work Out How Many Electrons An Element Has Each element is defined by the number of protons found in each of its atoms. The electronic structure of an atom can be predicted from its atomic number. The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after. Describe the locations, charges, and masses of the three main subatomic particles. No matter. How Do You Work Out How Many Electrons An Element Has.