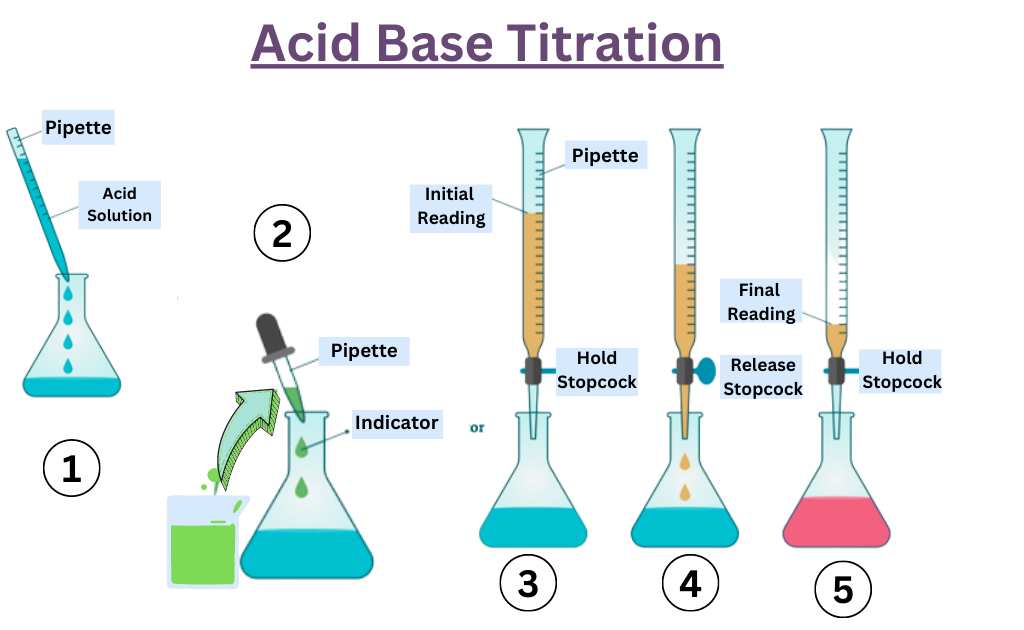
Titrations And Indicators . The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In the strong acid titration,. If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. This page assumes that you know about ph curves for all the. Titration is a common laboratory method of using quantitative chemical analysis. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration.
from themasterchemistry.com
This page assumes that you know about ph curves for all the. In the strong acid titration,. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. Titration is a common laboratory method of using quantitative chemical analysis. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration.
Acid Base TitrationWorking Principle, Process, Types And Indicators
Titrations And Indicators If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. Titration is a common laboratory method of using quantitative chemical analysis. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. This page assumes that you know about ph curves for all the. In the strong acid titration,. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a.
From www.vrogue.co
Titration Indicator Types Procedure Indicators vrogue.co Titrations And Indicators This page assumes that you know about ph curves for all the. Titration is a common laboratory method of using quantitative chemical analysis. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. The graph shows the results obtained using two indicators. Titrations And Indicators.
From stock.adobe.com
Acidbase titration and phenolphthalein indicator Stock Vector Adobe Titrations And Indicators Titration is a common laboratory method of using quantitative chemical analysis. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. This page assumes that. Titrations And Indicators.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Titrations And Indicators The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. This page assumes that you know about ph curves for all the. If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. The graph shows the results obtained using two indicators (methyl. Titrations And Indicators.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titrations And Indicators A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. This page assumes that you know about ph curves for all the. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows. Titrations And Indicators.
From saylordotorg.github.io
AcidBase Titrations Titrations And Indicators If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. Titration is a common laboratory method of using quantitative chemical analysis. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. This page assumes that you know. Titrations And Indicators.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titrations And Indicators The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. This page assumes that you know about ph curves for all the. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. Titration is a. Titrations And Indicators.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titrations And Indicators If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. Titration is a common laboratory method of using quantitative chemical analysis. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration.. Titrations And Indicators.
From www.science-revision.co.uk
Titrations Titrations And Indicators Titration is a common laboratory method of using quantitative chemical analysis. This page assumes that you know about ph curves for all the. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the. Titrations And Indicators.
From www.slideshare.net
Acid base titration Titrations And Indicators The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. In the strong acid titration,. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. Titration is a common laboratory. Titrations And Indicators.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titrations And Indicators This page assumes that you know about ph curves for all the. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. Titration is a common laboratory method of using quantitative. Titrations And Indicators.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titrations And Indicators Titration is a common laboratory method of using quantitative chemical analysis. If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In the strong acid titration,. A quantitative and volumetric technique, to. Titrations And Indicators.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titrations And Indicators If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In the strong acid titration,. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of. Titrations And Indicators.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titrations And Indicators If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the. Titrations And Indicators.
From byjus.com
Study The pH Change In The Titration Of A Strong Base Using Universal Titrations And Indicators This page assumes that you know about ph curves for all the. Titration is a common laboratory method of using quantitative chemical analysis. If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration. Titrations And Indicators.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titrations And Indicators The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. This page assumes that you know about ph curves for all the. In the strong acid titration,. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. If a large amount. Titrations And Indicators.
From theedge.com.hk
Chemistry How To Titration The Edge Titrations And Indicators A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. Titration is a common laboratory method of using quantitative chemical analysis. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of. Titrations And Indicators.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Titrations And Indicators In the strong acid titration,. This page assumes that you know about ph curves for all the. Titration is a common laboratory method of using quantitative chemical analysis. If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. The titration curves shown in figure 14.20 illustrate the choice of. Titrations And Indicators.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titrations And Indicators In the strong acid titration,. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. The titration curves shown in figure 14.20 illustrate the choice of a. Titrations And Indicators.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titrations And Indicators This page assumes that you know about ph curves for all the. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. A quantitative and volumetric technique, to determine the unknown. Titrations And Indicators.
From mmerevise.co.uk
Titrations and Uncertainties MME Titrations And Indicators If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. This page assumes that you know about ph curves for all the. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. A quantitative and volumetric technique, to determine the unknown concentration. Titrations And Indicators.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Titrations And Indicators A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. This page assumes that you know about ph curves for all the. If a large. Titrations And Indicators.
From mungfali.com
Acid Base Titration Indicator Titrations And Indicators The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Titration is a common laboratory method of using quantitative chemical analysis. This page assumes that you know about ph curves for. Titrations And Indicators.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titrations And Indicators A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. This page assumes that you know about ph curves for all the. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows. Titrations And Indicators.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titrations And Indicators Titration is a common laboratory method of using quantitative chemical analysis. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. This page assumes that you know about ph curves for all the. The titration curves shown in figure 14.20 illustrate the. Titrations And Indicators.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive Titrations And Indicators A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Titrations And Indicators.
From freesvg.org
Redox Titration Using Indicator Free SVG Titrations And Indicators The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the. Titrations And Indicators.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titrations And Indicators In the strong acid titration,. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. This page assumes that you. Titrations And Indicators.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titrations And Indicators The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. This page assumes that you know about ph curves for all the. In the strong. Titrations And Indicators.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Titrations And Indicators In the strong acid titration,. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. If a large amount of. Titrations And Indicators.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE Titrations And Indicators In the strong acid titration,. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration. Titrations And Indicators.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Titrations And Indicators The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. This page assumes that you know about ph curves for all the. In the strong acid titration,. If a large amount. Titrations And Indicators.
From www.sliderbase.com
Titration Titrations And Indicators The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Titration is a common laboratory method of using quantitative chemical analysis. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. If a large amount. Titrations And Indicators.
From jackwestin.com
Indicators Titration MCAT Content Titrations And Indicators If a large amount of indicator is used, the indicator will effect the final ph, lowering the accuracy of the experiment. In the strong acid titration,. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. The titration curves shown in figure 14.20 illustrate the choice of a. Titrations And Indicators.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID9234620 Titrations And Indicators The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Titrations And Indicators.
From courses.lumenlearning.com
AcidBase Titrations Boundless Chemistry Titrations And Indicators A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of indicator is called titration. This page assumes that you know about ph curves for all the. In the strong acid titration,. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Titrations And Indicators.