Bases In The Laboratory . A base that can dissolve. Acids and bases can neutralise each other. In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds, termed acids and bases. A base that can dissolve. Bases, on the other hand, are substances that accept protons or donate electron pairs. Acids and bases can neutralise each other. They turn blue litmus solution to red colour. Acids, bases and alkalis are found in the laboratory and at home. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide,. To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with the following: Chemical species which donate protons or release h + ions when dissolved in water are called acid. It is also commonly referred to as any substance that can react. Acids, bases and alkalis are found in the laboratory and at home. When dissolved in an aqueous solution, certain ions were released into the solution.
from www.dreamstime.com
To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with the following: Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide,. Acids and bases can neutralise each other. Bases, on the other hand, are substances that accept protons or donate electron pairs. They turn blue litmus solution to red colour. Acids and bases can neutralise each other. Acids, bases and alkalis are found in the laboratory and at home. Acids, bases and alkalis are found in the laboratory and at home. When dissolved in an aqueous solution, certain ions were released into the solution. It is also commonly referred to as any substance that can react.
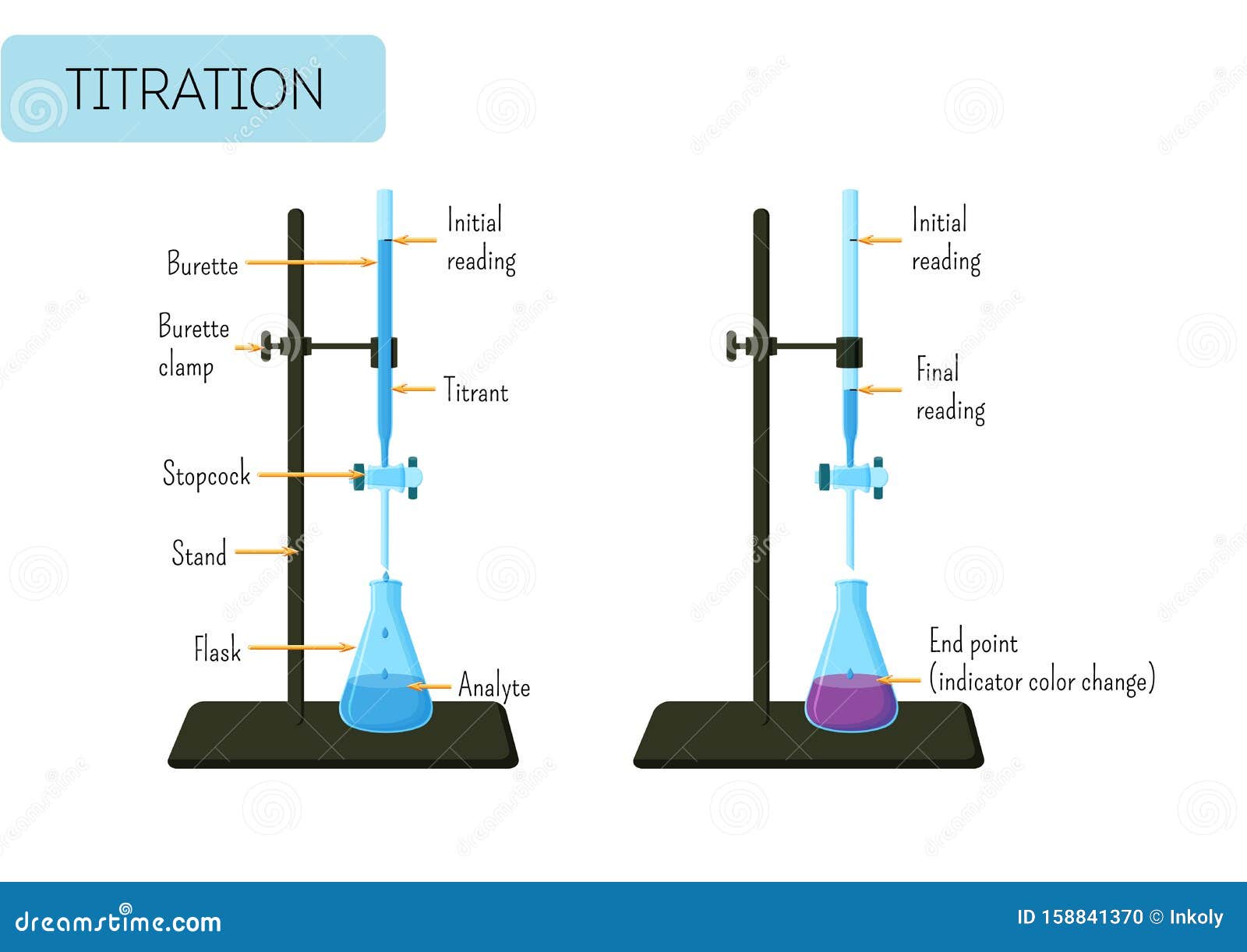
Laboratory Experiment of Acid Base Titration with Glass Burette and
Bases In The Laboratory A base that can dissolve. Chemical species which donate protons or release h + ions when dissolved in water are called acid. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide,. Acids and bases can neutralise each other. A base that can dissolve. When dissolved in an aqueous solution, certain ions were released into the solution. Acids and bases can neutralise each other. Bases, on the other hand, are substances that accept protons or donate electron pairs. Acids, bases and alkalis are found in the laboratory and at home. They turn blue litmus solution to red colour. A base that can dissolve. To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with the following: In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds, termed acids and bases. It is also commonly referred to as any substance that can react. Acids, bases and alkalis are found in the laboratory and at home.
From dissolve.com
Team of Research Scientists Have Meeting, They Have Discussion While Bases In The Laboratory Acids and bases can neutralise each other. Acids, bases and alkalis are found in the laboratory and at home. It is also commonly referred to as any substance that can react. Acids and bases can neutralise each other. A base that can dissolve. Chemical species which donate protons or release h + ions when dissolved in water are called acid.. Bases In The Laboratory.
From www.open.ac.uk
SS001 Laboratory Skills for Science OpenPlus Open University Bases In The Laboratory A base that can dissolve. Acids and bases can neutralise each other. Acids, bases and alkalis are found in the laboratory and at home. When dissolved in an aqueous solution, certain ions were released into the solution. It is also commonly referred to as any substance that can react. Acids and bases can neutralise each other. In 1884, the swedish. Bases In The Laboratory.
From www.dreamstime.com
View of a Group of Bases for Test Tubes in a Pharmaceutical Lab Stock Bases In The Laboratory A base that can dissolve. To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with the following: Acids, bases and alkalis are found in the laboratory and at home. Acids and bases can neutralise each other. Bases, on the other hand, are substances that accept protons or donate electron pairs. They turn blue litmus. Bases In The Laboratory.
From www.dreamstime.com
School Chemistry Laboratory Dye for Acids and Bases Stock Photo Bases In The Laboratory Acids and bases can neutralise each other. A base that can dissolve. They turn blue litmus solution to red colour. Acids and bases can neutralise each other. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide,. Acids, bases and alkalis are found in the laboratory and at home. To study the properties of acids (dilute hcl) and bases (dilute. Bases In The Laboratory.
From www.bu.edu
Laboratory » Chemistry Boston University Bases In The Laboratory When dissolved in an aqueous solution, certain ions were released into the solution. A base that can dissolve. Bases, on the other hand, are substances that accept protons or donate electron pairs. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide,. A base that can dissolve. Acids and bases can neutralise each other. They turn blue litmus solution to. Bases In The Laboratory.
From www.dreamstime.com
Laboratory Experiment of Acid Base Titration with Glass Burette and Bases In The Laboratory They turn blue litmus solution to red colour. A base that can dissolve. To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with the following: Chemical species which donate protons or release h + ions when dissolved in water are called acid. It is also commonly referred to as any substance that can react.. Bases In The Laboratory.
From crotraining.co.uk
What is the importance of Good Laboratory Practice? Bases In The Laboratory Bases, on the other hand, are substances that accept protons or donate electron pairs. Acids, bases and alkalis are found in the laboratory and at home. When dissolved in an aqueous solution, certain ions were released into the solution. Acids and bases can neutralise each other. A base that can dissolve. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium. Bases In The Laboratory.
From mungfali.com
Acid Base Titration Lab Bases In The Laboratory In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds, termed acids and bases. Acids and bases can neutralise each other. Acids, bases and alkalis are found in the laboratory and at home. A base that can dissolve. When dissolved in an aqueous solution, certain ions were released into the solution. It is also commonly referred to. Bases In The Laboratory.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID1487777 Bases In The Laboratory Chemical species which donate protons or release h + ions when dissolved in water are called acid. Bases, on the other hand, are substances that accept protons or donate electron pairs. A base that can dissolve. In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds, termed acids and bases. Acids, bases and alkalis are found in. Bases In The Laboratory.
From chemistrytalk.org
Properties of Acids and Bases ChemTalk Bases In The Laboratory Acids, bases and alkalis are found in the laboratory and at home. Bases, on the other hand, are substances that accept protons or donate electron pairs. It is also commonly referred to as any substance that can react. In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds, termed acids and bases. A base that can dissolve.. Bases In The Laboratory.
From www.labster.com
Virtual Lab Acids and Bases (Principles) Virtual Lab Labster Bases In The Laboratory When dissolved in an aqueous solution, certain ions were released into the solution. Acids and bases can neutralise each other. Bases, on the other hand, are substances that accept protons or donate electron pairs. Acids and bases can neutralise each other. Acids, bases and alkalis are found in the laboratory and at home. Examples include baking soda (sodium bicarbonate, nahco₃),. Bases In The Laboratory.
From www.youtube.com
Acids and Bases in the Laboratory YouTube Bases In The Laboratory Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide,. They turn blue litmus solution to red colour. Acids and bases can neutralise each other. A base that can dissolve. In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds, termed acids and bases. To study the properties of acids (dilute hcl) and bases (dilute naoh) by. Bases In The Laboratory.
From www.dreamstime.com
Determining Acidbases in the Chemistry Laboratory Stock Photo Image Bases In The Laboratory Acids, bases and alkalis are found in the laboratory and at home. Acids and bases can neutralise each other. Acids, bases and alkalis are found in the laboratory and at home. A base that can dissolve. Bases, on the other hand, are substances that accept protons or donate electron pairs. A base that can dissolve. To study the properties of. Bases In The Laboratory.
From www.slideserve.com
PPT Class Work Acids, Bases and Alkalis PowerPoint Presentation, free Bases In The Laboratory Acids, bases and alkalis are found in the laboratory and at home. Bases, on the other hand, are substances that accept protons or donate electron pairs. To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with the following: They turn blue litmus solution to red colour. When dissolved in an aqueous solution, certain ions. Bases In The Laboratory.
From www.slideserve.com
PPT Acids and Bases (3) PowerPoint Presentation, free download ID Bases In The Laboratory They turn blue litmus solution to red colour. To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with the following: When dissolved in an aqueous solution, certain ions were released into the solution. Bases, on the other hand, are substances that accept protons or donate electron pairs. Acids, bases and alkalis are found in. Bases In The Laboratory.
From www.dreamstime.com
Litmus Paper Test of Acid and Base in Chemistry Laboratory Stock Vector Bases In The Laboratory When dissolved in an aqueous solution, certain ions were released into the solution. Acids, bases and alkalis are found in the laboratory and at home. To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with the following: Acids and bases can neutralise each other. Bases, on the other hand, are substances that accept protons. Bases In The Laboratory.
From www.youtube.com
Std.10 Science Chapter 2 Acids, Bases and Salts Video 3 Acids Bases In The Laboratory A base that can dissolve. When dissolved in an aqueous solution, certain ions were released into the solution. In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds, termed acids and bases. Acids, bases and alkalis are found in the laboratory and at home. To study the properties of acids (dilute hcl) and bases (dilute naoh) by. Bases In The Laboratory.
From libbyenvironmental.com
Fixed Base Laboratory Services » Libby Environmental, Inc. Bases In The Laboratory A base that can dissolve. In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds, termed acids and bases. When dissolved in an aqueous solution, certain ions were released into the solution. Acids and bases can neutralise each other. It is also commonly referred to as any substance that can react. Examples include baking soda (sodium bicarbonate,. Bases In The Laboratory.
From www.genengnews.com
Artificial IntelligenceBased Lab Partner Designs, Runs Experiments in Bases In The Laboratory Acids, bases and alkalis are found in the laboratory and at home. When dissolved in an aqueous solution, certain ions were released into the solution. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide,. Acids and bases can neutralise each other. A base that can dissolve. In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds,. Bases In The Laboratory.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2167333 Bases In The Laboratory Acids, bases and alkalis are found in the laboratory and at home. Acids and bases can neutralise each other. When dissolved in an aqueous solution, certain ions were released into the solution. Bases, on the other hand, are substances that accept protons or donate electron pairs. In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds, termed. Bases In The Laboratory.
From www.medpace.com
Global Central Laboratories Medpace Bases In The Laboratory Acids and bases can neutralise each other. A base that can dissolve. It is also commonly referred to as any substance that can react. In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds, termed acids and bases. Acids and bases can neutralise each other. Bases, on the other hand, are substances that accept protons or donate. Bases In The Laboratory.
From www.148apps.com
Acid and bases in laboratory Apps 148Apps Bases In The Laboratory Chemical species which donate protons or release h + ions when dissolved in water are called acid. Acids, bases and alkalis are found in the laboratory and at home. A base that can dissolve. To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with the following: Acids and bases can neutralise each other. Acids. Bases In The Laboratory.
From www.pinterest.com
Laboratory Basics Acids and bases Acids and bases are fun to learn Bases In The Laboratory Acids and bases can neutralise each other. Acids, bases and alkalis are found in the laboratory and at home. A base that can dissolve. When dissolved in an aqueous solution, certain ions were released into the solution. They turn blue litmus solution to red colour. Bases, on the other hand, are substances that accept protons or donate electron pairs. Acids,. Bases In The Laboratory.
From www.thoughtco.com
All You Need to Know About Acids, Bases, and pH Bases In The Laboratory Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide,. A base that can dissolve. Acids and bases can neutralise each other. A base that can dissolve. Chemical species which donate protons or release h + ions when dissolved in water are called acid. To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with. Bases In The Laboratory.
From www.flinnsci.com
AcidBase Test Kit I—Properties of Acids and Bases—Super Value Bases In The Laboratory It is also commonly referred to as any substance that can react. To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with the following: A base that can dissolve. In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds, termed acids and bases. A base that can dissolve. Acids and bases. Bases In The Laboratory.
From www.laboratorysciencecareers.com
Why Laboratory Science Is A Great Career Laboratory Science Careers Bases In The Laboratory A base that can dissolve. In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds, termed acids and bases. Chemical species which donate protons or release h + ions when dissolved in water are called acid. It is also commonly referred to as any substance that can react. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium. Bases In The Laboratory.
From pharmacyscope.com
Theories of Acids and Bases Pharmacy Scope Bases In The Laboratory When dissolved in an aqueous solution, certain ions were released into the solution. In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds, termed acids and bases. A base that can dissolve. To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with the following: They turn blue litmus solution to red. Bases In The Laboratory.
From studylib.net
ACIDS AND BASES Bases In The Laboratory Acids, bases and alkalis are found in the laboratory and at home. When dissolved in an aqueous solution, certain ions were released into the solution. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide,. Chemical species which donate protons or release h + ions when dissolved in water are called acid. They turn blue litmus solution to red colour.. Bases In The Laboratory.
From blog.praxilabs.com
Chemistry Experiments Virtual Labs How to Implement It Bases In The Laboratory Acids and bases can neutralise each other. In 1884, the swedish chemist svante arrhenius proposed two specific classifications of compounds, termed acids and bases. To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with the following: It is also commonly referred to as any substance that can react. They turn blue litmus solution to. Bases In The Laboratory.
From hayejemplos.com
Ejemplos de bases químicas Bases In The Laboratory Acids, bases and alkalis are found in the laboratory and at home. Chemical species which donate protons or release h + ions when dissolved in water are called acid. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide,. Acids and bases can neutralise each other. It is also commonly referred to as any substance that can react. Acids and. Bases In The Laboratory.
From www.flinnsci.com
Acid Base Test Kit II Chemistry Laboratory Kits Bases In The Laboratory Acids and bases can neutralise each other. Bases, on the other hand, are substances that accept protons or donate electron pairs. They turn blue litmus solution to red colour. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide,. It is also commonly referred to as any substance that can react. When dissolved in an aqueous solution, certain ions were. Bases In The Laboratory.
From www.dreamstime.com
Scientist Showing a Dewaxed Tissue Samples in the Laboratory. Xylene Bases In The Laboratory To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with the following: Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide,. Acids and bases can neutralise each other. When dissolved in an aqueous solution, certain ions were released into the solution. Acids, bases and alkalis are found in the laboratory and at home.. Bases In The Laboratory.
From www.youtube.com
Lab Demonstration Acid Base Titration. YouTube Bases In The Laboratory They turn blue litmus solution to red colour. Acids, bases and alkalis are found in the laboratory and at home. Acids, bases and alkalis are found in the laboratory and at home. It is also commonly referred to as any substance that can react. A base that can dissolve. A base that can dissolve. Acids and bases can neutralise each. Bases In The Laboratory.
From www.slideserve.com
PPT Chapter 13 Acids and Bases The Molecules Responsible for Sour Bases In The Laboratory Chemical species which donate protons or release h + ions when dissolved in water are called acid. Acids, bases and alkalis are found in the laboratory and at home. It is also commonly referred to as any substance that can react. Bases, on the other hand, are substances that accept protons or donate electron pairs. They turn blue litmus solution. Bases In The Laboratory.
From www.dreamstime.com
Ph Paper in Science Laboratory Indicates Acid and Bases Stock Photo Bases In The Laboratory Acids, bases and alkalis are found in the laboratory and at home. Acids, bases and alkalis are found in the laboratory and at home. To study the properties of acids (dilute hcl) and bases (dilute naoh) by their reactions with the following: A base that can dissolve. When dissolved in an aqueous solution, certain ions were released into the solution.. Bases In The Laboratory.