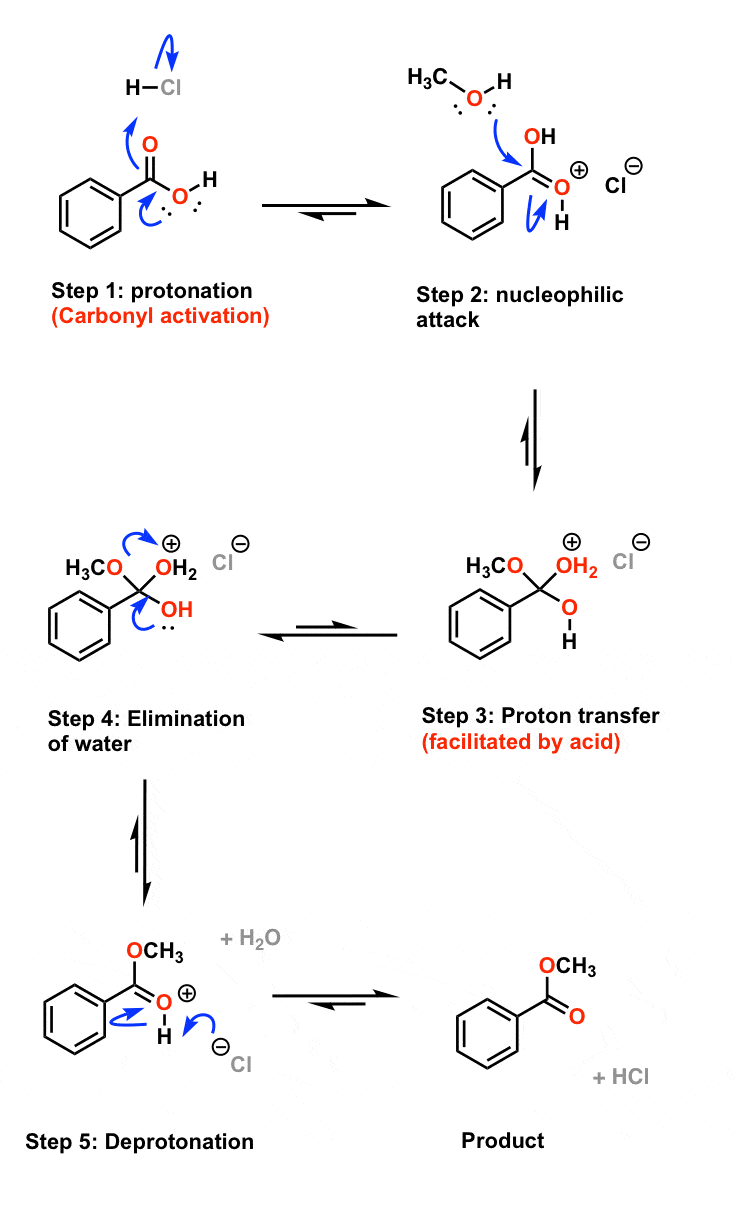
Catalyst Esterification Carboxylic Acid . The catalyst is usually concentrated. carboxylic acids can react with alcohols to form esters in a process called fischer esterification. When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water). The catalyst is usually concentrated. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. An acid catalyst is required. carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. This reaction is called the. typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as.
from www.masterorganicchemistry.com
The catalyst is usually concentrated. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as. typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water). esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated. carboxylic acids can react with alcohols to form esters in a process called fischer esterification. This reaction is called the. An acid catalyst is required.
Acid Catalysis of Organic Reactions Why It Works
Catalyst Esterification Carboxylic Acid esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. This reaction is called the. fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as. When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water). esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated. carboxylic acids can react with alcohols to form esters in a process called fischer esterification. The catalyst is usually concentrated. carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. An acid catalyst is required.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic Catalyst Esterification Carboxylic Acid esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. This reaction is called the. An acid catalyst is required. fischer esterification is the esterification of a carboxylic acid by heating it with. Catalyst Esterification Carboxylic Acid.
From www.chemistrysteps.com
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Catalyst Esterification Carboxylic Acid carboxylic acids can react with alcohols to form esters in a process called fischer esterification. carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. When a carboxylic acid is treated with an alcohol. Catalyst Esterification Carboxylic Acid.
From askfilo.com
Mechanism of esterification of carboxylic acids The esterification of ca.. Catalyst Esterification Carboxylic Acid carboxylic acids can react with alcohols to form esters in a process called fischer esterification. The catalyst is usually concentrated. An acid catalyst is required. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. carboxylic acids can be converted to esters with an acid catalyst and an excess of. Catalyst Esterification Carboxylic Acid.
From www.mdpi.com
Applied Sciences Free FullText Synthesis and Investigation of Catalyst Esterification Carboxylic Acid This reaction is called the. An acid catalyst is required. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. esters are produced when carboxylic acids are heated with alcohols in the presence of. Catalyst Esterification Carboxylic Acid.
From br.pinterest.com
Mechanism of AcidCatalyzed Hydrolysis of An Ester with a Tertiary Catalyst Esterification Carboxylic Acid carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as. When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with. Catalyst Esterification Carboxylic Acid.
From ar.inspiredpencil.com
Fischer Esterification Mechanism Carboxylic Acid Catalyst Esterification Carboxylic Acid An acid catalyst is required. typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as. This reaction is called the. esters are produced when carboxylic acids are heated. Catalyst Esterification Carboxylic Acid.
From www.thesciencehive.co.uk
Alcohols, Carboxylic Acids and Esters (GCSE) — the science hive Catalyst Esterification Carboxylic Acid When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water). typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. This reaction is called the.. Catalyst Esterification Carboxylic Acid.
From www.animalia-life.club
Esterification Mechanism Catalyst Esterification Carboxylic Acid esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. An acid catalyst is required. carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. carboxylic acids can react with alcohols to form esters in a process called fischer esterification. fischer esterification is. Catalyst Esterification Carboxylic Acid.
From www.organic-chemistry.org
Protic Acid Immobilized on Solid Support as an Extremely Efficient Catalyst Esterification Carboxylic Acid carboxylic acids can react with alcohols to form esters in a process called fischer esterification. carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. The catalyst is usually concentrated. typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. An acid catalyst is required.. Catalyst Esterification Carboxylic Acid.
From nrochemistry.com
Fischer Esterification Catalyst Esterification Carboxylic Acid esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated. typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water).. Catalyst Esterification Carboxylic Acid.
From www.masterorganicchemistry.com
Acid Catalysis of Organic Reactions Why It Works Catalyst Esterification Carboxylic Acid esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated. An acid catalyst is required. This reaction is called the. When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water). typical fisher esterification reaction involves heating. Catalyst Esterification Carboxylic Acid.
From chemistryguru.com.sg
Carboxylic Acid Reactions Organic Chem Catalyst Esterification Carboxylic Acid esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as. carboxylic acids can react with alcohols to form esters in a process called fischer esterification. esters. Catalyst Esterification Carboxylic Acid.
From www.researchgate.net
Scheme 2 Probable mechanism for an acid catalyzed esterification of a Catalyst Esterification Carboxylic Acid When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water). The catalyst is usually concentrated. The catalyst is usually concentrated. This reaction is called the. carboxylic acids can react with alcohols to form esters in a process called fischer esterification. fischer esterification is the esterification of a carboxylic. Catalyst Esterification Carboxylic Acid.
From www.researchgate.net
Mechanism of lipase in transesterification. Download Scientific Diagram Catalyst Esterification Carboxylic Acid typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. The catalyst is usually concentrated. carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. carboxylic acids can react with alcohols to form esters in a process called fischer esterification. This reaction is called the.. Catalyst Esterification Carboxylic Acid.
From dail-dd.blogspot.com
Carboxylic Acid And Alcohol / Solved Name The Carboxylic Acid And The Catalyst Esterification Carboxylic Acid When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water). carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. The catalyst is usually concentrated. An acid catalyst is required. carboxylic acids can react with alcohols to form esters in a process. Catalyst Esterification Carboxylic Acid.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic Catalyst Esterification Carboxylic Acid carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated. fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of. Catalyst Esterification Carboxylic Acid.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic Catalyst Esterification Carboxylic Acid This reaction is called the. The catalyst is usually concentrated. fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as. An acid catalyst is required. When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water). . Catalyst Esterification Carboxylic Acid.
From pubs.acs.org
Chemoselective Catalytic αOxidation of Carboxylic Acids Iron/Alkali Catalyst Esterification Carboxylic Acid An acid catalyst is required. typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water).. Catalyst Esterification Carboxylic Acid.
From www.chemistrysteps.com
Fischer Esterification Chemistry Steps Catalyst Esterification Carboxylic Acid When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water). esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. This reaction is called the. carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol.. Catalyst Esterification Carboxylic Acid.
From www.youtube.com
08.08 Esterification of Carboxylic Acids YouTube Catalyst Esterification Carboxylic Acid typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. This reaction is called the. An acid catalyst is required. The catalyst is usually concentrated. When a carboxylic acid is treated with an alcohol and an. Catalyst Esterification Carboxylic Acid.
From www.researchgate.net
Proposed reaction mechanism of stearic acid esterification over the Catalyst Esterification Carboxylic Acid esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated. This reaction is called the. fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as. carboxylic acids can be converted to esters. Catalyst Esterification Carboxylic Acid.
From www.masterorganicchemistry.com
Conversion of carboxylic acids to esters using acid and alcohols Catalyst Esterification Carboxylic Acid typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. carboxylic acids can react with alcohols to form esters in a process called fischer esterification. fischer esterification is the esterification of a carboxylic. Catalyst Esterification Carboxylic Acid.
From www.slideserve.com
PPT Chap 18. Carboxylic acids and their derivatives. Nucleophilic Catalyst Esterification Carboxylic Acid typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. This reaction is called the. carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. fischer esterification. Catalyst Esterification Carboxylic Acid.
From www.masterorganicchemistry.com
Conversion of carboxylic acids to esters using acid and alcohols Catalyst Esterification Carboxylic Acid fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as. The catalyst is usually concentrated. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. typical fisher esterification reaction involves heating a mixture of carboxylic acids and. Catalyst Esterification Carboxylic Acid.
From www.researchgate.net
Acidcatalyzed esterification. Reaction conditions and yields for Catalyst Esterification Carboxylic Acid When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water). carboxylic acids can react with alcohols to form esters in a process called fischer esterification. This reaction is called the. fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence. Catalyst Esterification Carboxylic Acid.
From www.researchgate.net
Esterification of carboxylic acid derivatives using supported iron Catalyst Esterification Carboxylic Acid When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water). typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. The catalyst is usually concentrated. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst.. Catalyst Esterification Carboxylic Acid.
From pdfprof.com
nucleophilic acyl substitution practice problems Catalyst Esterification Carboxylic Acid esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated. typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. This reaction is called the. carboxylic acids can be converted to esters with an acid catalyst and an excess. Catalyst Esterification Carboxylic Acid.
From pubs.acs.org
Direct Lewis Acid Catalyzed Conversion of Enantioenriched N Catalyst Esterification Carboxylic Acid carboxylic acids can react with alcohols to form esters in a process called fischer esterification. An acid catalyst is required. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated. fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol. Catalyst Esterification Carboxylic Acid.
From www.scientific.net
A Simple Procedure for the Esterification and Transesterification Using Catalyst Esterification Carboxylic Acid carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated. This reaction is called the. typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess. Catalyst Esterification Carboxylic Acid.
From www.researchgate.net
Esterification of some carboxylic acids with DMC under catalysis of K 2 Catalyst Esterification Carboxylic Acid esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as. carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. An. Catalyst Esterification Carboxylic Acid.
From www.chemistrysteps.com
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Catalyst Esterification Carboxylic Acid carboxylic acids can react with alcohols to form esters in a process called fischer esterification. This reaction is called the. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. An acid catalyst is required. The catalyst is usually concentrated. fischer esterification is the esterification of a carboxylic acid by. Catalyst Esterification Carboxylic Acid.
From www.researchgate.net
(PDF) Diphenylammonium triflate (DPAT) Efficient catalyst for Catalyst Esterification Carboxylic Acid fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid as. The catalyst is usually concentrated. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated. An acid catalyst is required. When a carboxylic. Catalyst Esterification Carboxylic Acid.
From revise.im
Carboxylic Acids and Esters Revise.im Catalyst Esterification Carboxylic Acid When a carboxylic acid is treated with an alcohol and an acid catalyst, an ester is formed (along with water). esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. typical fisher esterification reaction involves heating a mixture of carboxylic acids and an excess amount of. The catalyst is usually concentrated.. Catalyst Esterification Carboxylic Acid.
From www.sarthaks.com
Explain the mechanism of esterification carboxylic acids. Sarthaks Catalyst Esterification Carboxylic Acid esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. This reaction is called the. carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst. Catalyst Esterification Carboxylic Acid.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic Catalyst Esterification Carboxylic Acid carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. An acid catalyst is required. esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated. esters are produced when carboxylic acids are heated with alcohols in the presence of. Catalyst Esterification Carboxylic Acid.