Copper Ii Sulfate And Sodium Hydroxide . Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. Sodium hydroxide precipitates copper (ii) hydroxide: One mole of copper (ii) sulfate. The result is a blue precipitate. Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). (duration 7 seconds, size 401 k, file. Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water.
from solvedlib.com
The result is a blue precipitate. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. (duration 7 seconds, size 401 k, file. Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). One mole of copper (ii) sulfate.
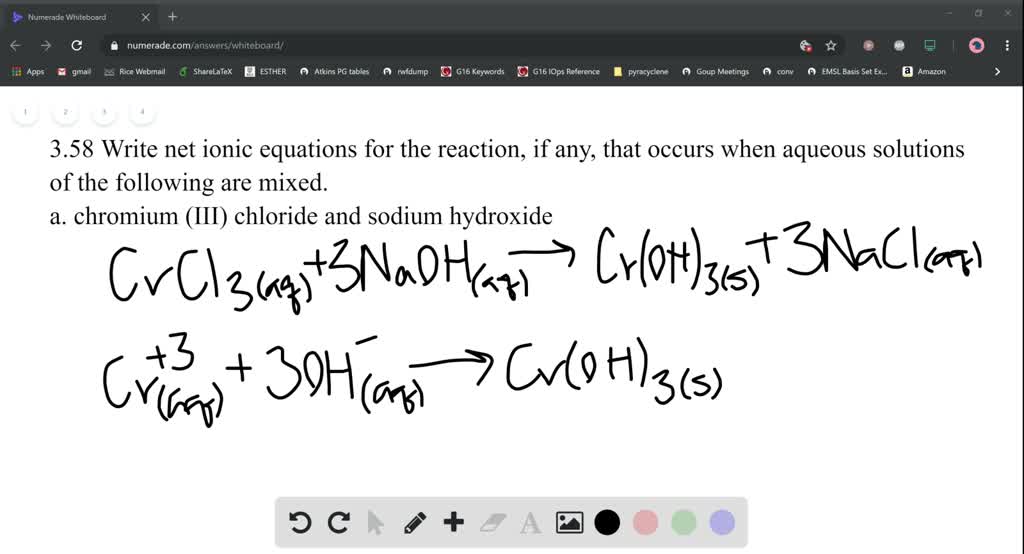
Write net ionic equations for the reaction, if any, t… SolvedLib
Copper Ii Sulfate And Sodium Hydroxide Use the calculator below to balance chemical equations and determine the type of reaction (instructions). One mole of copper (ii) sulfate. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. The result is a blue precipitate. Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. (duration 7 seconds, size 401 k, file. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Sodium hydroxide precipitates copper (ii) hydroxide:
From www.youtube.com
Copper(II) Sulfate and Sodium Hydroxide reaction CuSO4 + NaOH Copper Ii Sulfate And Sodium Hydroxide Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Copper (ii) sulfate pentahydrate + sodium. Copper Ii Sulfate And Sodium Hydroxide.
From www.dreamstime.com
Interaction of Solutions of Sodium Hydroxide and Copper II Sulfate. the Copper Ii Sulfate And Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. Sodium hydroxide precipitates copper (ii) hydroxide: Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. Use either a net ionic. Copper Ii Sulfate And Sodium Hydroxide.
From www.reddit.com
Why is the answer D and not C? Copper(II) sulfate is soluble in water Copper Ii Sulfate And Sodium Hydroxide I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Sodium hydroxide precipitates copper (ii) hydroxide: Copper (ii) sulfate. Copper Ii Sulfate And Sodium Hydroxide.
From answerhappy.com
A chemist prepares a solution of copper(II) sulfate (CuSO4) by Copper Ii Sulfate And Sodium Hydroxide Use the calculator below to balance chemical equations and determine the type of reaction (instructions). One mole of copper (ii) sulfate. (duration 7 seconds, size 401 k, file. The result is a blue precipitate. Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. Sodium hydroxide precipitates copper (ii) hydroxide: Use either a net ionic. Copper Ii Sulfate And Sodium Hydroxide.
From www.chegg.com
Solved Suppose that you repeated the reaction between Copper Ii Sulfate And Sodium Hydroxide Use the calculator below to balance chemical equations and determine the type of reaction (instructions). I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. Here, copper (ii) sulfate (cuso 4) is added to sodium. Copper Ii Sulfate And Sodium Hydroxide.
From www.istockphoto.com
Copper Sulfate And Sodium Hydroxide Pellets In Test Tube With Plug Cap Copper Ii Sulfate And Sodium Hydroxide The result is a blue precipitate. (duration 7 seconds, size 401 k, file. Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). One mole of copper (ii) sulfate. Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with. Copper Ii Sulfate And Sodium Hydroxide.
From www.chegg.com
Solved copper (II) sulfate + sodium hydroxide → Blue Copper Ii Sulfate And Sodium Hydroxide The result is a blue precipitate. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. (duration 7 seconds, size 401 k, file. One mole of copper (ii) sulfate. Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. Use the calculator below to balance chemical equations and. Copper Ii Sulfate And Sodium Hydroxide.
From www.alamy.com
Copper(II) sulfate with molecular structure. Chemical ingredient used Copper Ii Sulfate And Sodium Hydroxide Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Sodium hydroxide precipitates copper (ii) hydroxide: (duration 7 seconds, size 401 k, file. 7 rows solutions. Copper Ii Sulfate And Sodium Hydroxide.
From www.youtube.com
Amino Acids 3. Reactions with sodium hydroxide, sodium carbonate Copper Ii Sulfate And Sodium Hydroxide The result is a blue precipitate. Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. I tried reacting copper sulfate with sodium hydroxide to get. Copper Ii Sulfate And Sodium Hydroxide.
From www.youtube.com
Sodium Hydroxide and Copper Sulfate reaction YouTube Copper Ii Sulfate And Sodium Hydroxide I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. Use the calculator below. Copper Ii Sulfate And Sodium Hydroxide.
From brainly.com
When copper sulfate reacts with sodium hydroxide solution, a Copper Ii Sulfate And Sodium Hydroxide Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. 7 rows solutions containing. Copper Ii Sulfate And Sodium Hydroxide.
From cartoondealer.com
Sodium Hydroxide Pellets, Zinc Granules And Copper(II) Sulfate In Test Copper Ii Sulfate And Sodium Hydroxide One mole of copper (ii) sulfate. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Use either. Copper Ii Sulfate And Sodium Hydroxide.
From www.youtube.com
Copper Sulfate and Sodium Hydroxide YouTube Copper Ii Sulfate And Sodium Hydroxide One mole of copper (ii) sulfate. (duration 7 seconds, size 401 k, file. Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Sodium hydroxide + copper (ii). Copper Ii Sulfate And Sodium Hydroxide.
From www.alamy.com
Chemical ingredient in hexagonal molecular shaped container. Sodium Copper Ii Sulfate And Sodium Hydroxide Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. The result is a blue precipitate. Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. Sodium hydroxide precipitates copper (ii) hydroxide: Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh).. Copper Ii Sulfate And Sodium Hydroxide.
From www.youtube.com
Reaction of Sodium Hydroxide and Copper Sulfate YouTube Copper Ii Sulfate And Sodium Hydroxide Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. Sodium hydroxide precipitates copper (ii) hydroxide: I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. Use either. Copper Ii Sulfate And Sodium Hydroxide.
From www.dreamstime.com
Sodium Hydroxide Pellets, Zinc Granules and Copper(II) Sulfate in Test Copper Ii Sulfate And Sodium Hydroxide One mole of copper (ii) sulfate. Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Sodium hydroxide precipitates copper (ii) hydroxide: Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Use either a net. Copper Ii Sulfate And Sodium Hydroxide.
From www.alamy.com
Zinc Powder, Copper(II) sulfate and Sodium Hydroxide Pellets in test Copper Ii Sulfate And Sodium Hydroxide Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. One mole of copper (ii) sulfate. (duration 7 seconds, size 401 k, file. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). The. Copper Ii Sulfate And Sodium Hydroxide.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Ii Sulfate And Sodium Hydroxide Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. Sodium hydroxide precipitates copper (ii) hydroxide: The result is a blue precipitate. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the. Copper Ii Sulfate And Sodium Hydroxide.
From www.deperu.com
Reacción de doble desplazamiento hidróxido de sodio y sulfato de cobre Copper Ii Sulfate And Sodium Hydroxide Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. The result is a blue precipitate. One mole of copper (ii) sulfate. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full). Copper Ii Sulfate And Sodium Hydroxide.
From fphoto.photoshelter.com
science chemistry compound cupric sulfate Fundamental Photographs Copper Ii Sulfate And Sodium Hydroxide (duration 7 seconds, size 401 k, file. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Sodium hydroxide precipitates copper (ii) hydroxide: The result is a blue precipitate. Use the calculator below to balance chemical equations and. Copper Ii Sulfate And Sodium Hydroxide.
From oneclass.com
OneClass Write the balanced net ionic equation for the reactions that Copper Ii Sulfate And Sodium Hydroxide One mole of copper (ii) sulfate. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. I tried reacting copper sulfate with sodium hydroxide to get. Copper Ii Sulfate And Sodium Hydroxide.
From brainly.in
What are the products of Copper sulphate + sodium hydroxide? Brainly.in Copper Ii Sulfate And Sodium Hydroxide (duration 7 seconds, size 401 k, file. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. The result is a blue precipitate. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation). Copper Ii Sulfate And Sodium Hydroxide.
From www.chegg.com
Solved Copper(II) sulfate + Sodium hydroxide Blue gelatinous Copper Ii Sulfate And Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. The result is a blue precipitate. Sodium hydroxide precipitates copper (ii) hydroxide: Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic.. Copper Ii Sulfate And Sodium Hydroxide.
From www.reddit.com
Copper(II) sulfate r/MineralPorn Copper Ii Sulfate And Sodium Hydroxide I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. The result is a blue precipitate. Use either. Copper Ii Sulfate And Sodium Hydroxide.
From cupsoguepictures.com
️ Hydrated copper sulfate. What Is the Formula for Copper(II) Sulfate Copper Ii Sulfate And Sodium Hydroxide One mole of copper (ii) sulfate. Sodium hydroxide precipitates copper (ii) hydroxide: Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. (duration 7 seconds, size 401 k, file. Use either a net ionic equations (omit the na+), molecular equation (include the copper. Copper Ii Sulfate And Sodium Hydroxide.
From www.alamy.com
Chemical ingredient in hexagonal molecular shaped container. Hair Copper Ii Sulfate And Sodium Hydroxide I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. Here, copper (ii) sulfate. Copper Ii Sulfate And Sodium Hydroxide.
From nghenhansu.edu.vn
Top 94+ Images Sodium Hydroxide And Copper(ii) Nitrate Sharp Copper Ii Sulfate And Sodium Hydroxide Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Sodium hydroxide precipitates copper (ii) hydroxide: I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. The result is a. Copper Ii Sulfate And Sodium Hydroxide.
From www.alamy.com
Chemical ingredient in hexagonal molecular shaped container. Sodium Copper Ii Sulfate And Sodium Hydroxide Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). The result is a blue precipitate. Sodium hydroxide precipitates copper (ii) hydroxide: One mole of copper (ii) sulfate. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. (duration 7 seconds, size 401 k, file. Use the calculator below to balance. Copper Ii Sulfate And Sodium Hydroxide.
From crystalverse.com
The Best Way to Grow Big Copper Sulfate Crystals Crystalverse Copper Ii Sulfate And Sodium Hydroxide I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Copper (ii) sulfate pentahydrate + sodium hydroxide =. Copper Ii Sulfate And Sodium Hydroxide.
From www.alamy.com
Chemical ingredient in hexagonal molecular shaped container. Urea Copper Ii Sulfate And Sodium Hydroxide (duration 7 seconds, size 401 k, file. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). The result is a blue precipitate. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Sodium hydroxide precipitates copper (ii) hydroxide: Sodium hydroxide + copper (ii) sulfate. Copper Ii Sulfate And Sodium Hydroxide.
From www.storyblocks.com
Interaction Of Solutions Of Sodium Hydroxide Stock Footage SBV Copper Ii Sulfate And Sodium Hydroxide One mole of copper (ii) sulfate. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium. Copper Ii Sulfate And Sodium Hydroxide.
From www.chegg.com
Solved copper (II) sulfate + sodium hydroxide → Blue Copper Ii Sulfate And Sodium Hydroxide Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). One mole of copper (ii) sulfate. Sodium hydroxide precipitates copper (ii) hydroxide: Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. (duration 7 seconds, size. Copper Ii Sulfate And Sodium Hydroxide.
From www.shimico.com
Copper Sulfate and the methods of production Shimico blog Copper Ii Sulfate And Sodium Hydroxide One mole of copper (ii) sulfate. Sodium hydroxide + copper (ii) sulfate → balanced chemical equation (molecular equation) lonic equation net ionic equation. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Copper (ii) sulfate pentahydrate + sodium hydroxide = cupric hydroxide + sodium sulfate + water. Here, copper. Copper Ii Sulfate And Sodium Hydroxide.
From martha-has-shaw.blogspot.com
Molecular Equation for Copper Ii Sulfate Sodium Carbonate MarthahasShaw Copper Ii Sulfate And Sodium Hydroxide The result is a blue precipitate. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or complete ionic. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Copper (ii) sulfate pentahydrate + sodium hydroxide =. Copper Ii Sulfate And Sodium Hydroxide.
From solvedlib.com
Write net ionic equations for the reaction, if any, t… SolvedLib Copper Ii Sulfate And Sodium Hydroxide 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. I tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. (duration 7 seconds, size 401 k, file. Use either a net ionic equations (omit the na+), molecular equation (include the copper compound full). Copper Ii Sulfate And Sodium Hydroxide.