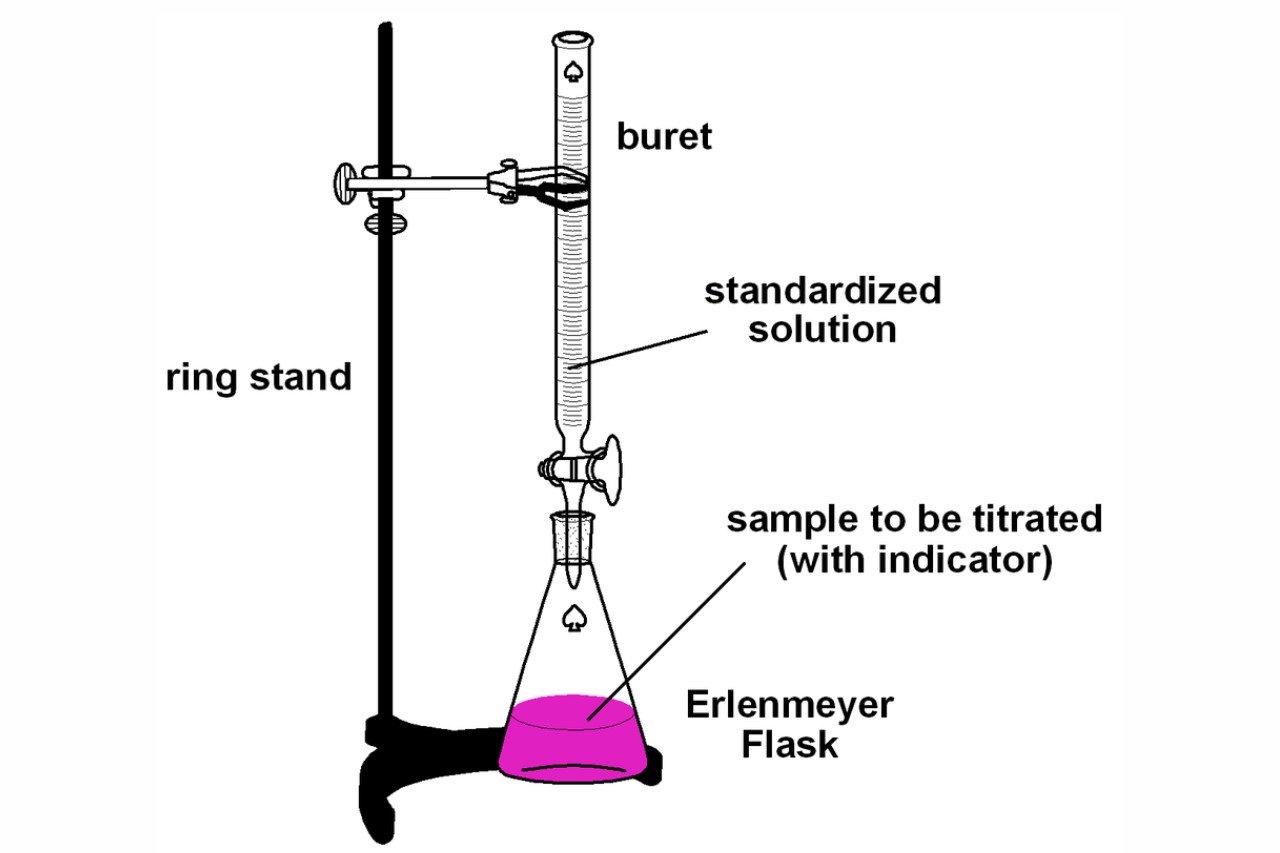
Indicator Of Acid Base Titration . The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. The reagent (titrant) is the solution with a The analyte (titrand) is the solution with an unknown molarity. The synthesis of organic dyes provided many new indicators. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. This page assumes that you know. How do acid base indicators work?
from facts.net
How do acid base indicators work? The synthesis of organic dyes provided many new indicators. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. The reagent (titrant) is the solution with a The analyte (titrand) is the solution with an unknown molarity. This page assumes that you know. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh.
8 Captivating Facts About AcidBase Titration
Indicator Of Acid Base Titration This page assumes that you know. This page assumes that you know. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. The synthesis of organic dyes provided many new indicators. The reagent (titrant) is the solution with a In this article, the author has explained acid base titration, its working principle types and process of acid base titration. The analyte (titrand) is the solution with an unknown molarity. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. How do acid base indicators work?
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Indicator Of Acid Base Titration The synthesis of organic dyes provided many new indicators. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid). Indicator Of Acid Base Titration.
From atlas.nilebasin.org
Acidbase Titration Setup Phenolphthalein Indicator Vector, 42 OFF Indicator Of Acid Base Titration In this article, the author has explained acid base titration, its working principle types and process of acid base titration. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. The reagent (titrant) is the solution with a The synthesis of organic dyes provided many new indicators.. Indicator Of Acid Base Titration.
From ar.inspiredpencil.com
Acid Base Titration Indicator Of Acid Base Titration This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown.. Indicator Of Acid Base Titration.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint Indicator Of Acid Base Titration This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown.. Indicator Of Acid Base Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Indicator Of Acid Base Titration In this article, the author has explained acid base titration, its working principle types and process of acid base titration. The analyte (titrand) is the solution with an unknown molarity. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. How do acid base indicators work? The. Indicator Of Acid Base Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicator Of Acid Base Titration In this article, the author has explained acid base titration, its working principle types and process of acid base titration. How do acid base indicators work? This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with. Indicator Of Acid Base Titration.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry Indicator Of Acid Base Titration This page assumes that you know. The synthesis of organic dyes provided many new indicators. How do acid base indicators work? This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. The ph. Indicator Of Acid Base Titration.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle Indicator Of Acid Base Titration The reagent (titrant) is the solution with a This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. In this article, the author has explained acid base titration, its working principle types and. Indicator Of Acid Base Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Indicator Of Acid Base Titration In this article, the author has explained acid base titration, its working principle types and process of acid base titration. The synthesis of organic dyes provided many new indicators. This page assumes that you know. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong. Indicator Of Acid Base Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Indicator Of Acid Base Titration The reagent (titrant) is the solution with a In this article, the author has explained acid base titration, its working principle types and process of acid base titration. This page assumes that you know. The synthesis of organic dyes provided many new indicators. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml. Indicator Of Acid Base Titration.
From www.slideshare.net
Acid base titration Indicator Of Acid Base Titration This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. The synthesis of organic dyes provided many new indicators. This page assumes that you know. How do acid base indicators work? The ph. Indicator Of Acid Base Titration.
From www.hotzxgirl.com
Solved Choose The Correct Indicator For Acid Base Titration Chegg Hot Indicator Of Acid Base Titration The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. The reagent (titrant) is the solution with a The analyte (titrand) is the solution with an unknown molarity. This page assumes that you know. This figure shows plots of ph versus volume of base added for the. Indicator Of Acid Base Titration.
From mmerevise.co.uk
Titrations and Uncertainties MME Indicator Of Acid Base Titration The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. The reagent (titrant) is the solution with a This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a. Indicator Of Acid Base Titration.
From facts.net
8 Captivating Facts About AcidBase Titration Indicator Of Acid Base Titration The synthesis of organic dyes provided many new indicators. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. This page assumes that you know. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a In this article, the. Indicator Of Acid Base Titration.
From mungfali.com
Acid Base Titration Indicator Indicator Of Acid Base Titration In this article, the author has explained acid base titration, its working principle types and process of acid base titration. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. The reagent (titrant) is the solution with a The analyte (titrand) is the solution with an unknown. Indicator Of Acid Base Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicator Of Acid Base Titration The reagent (titrant) is the solution with a This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. In this article, the author has explained acid base titration, its working principle types and. Indicator Of Acid Base Titration.
From stock.adobe.com
Acidbase titration and phenolphthalein indicator Stock Vector Adobe Indicator Of Acid Base Titration In this article, the author has explained acid base titration, its working principle types and process of acid base titration. The analyte (titrand) is the solution with an unknown molarity. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. How do acid base indicators work? This. Indicator Of Acid Base Titration.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Indicator Of Acid Base Titration The synthesis of organic dyes provided many new indicators. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. This page assumes that you know. The reagent (titrant) is the solution with a This figure shows plots of ph versus volume of base added for the titration of 50.0 ml. Indicator Of Acid Base Titration.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Indicator Of Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. How do acid base indicators work? The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m. Indicator Of Acid Base Titration.
From mungfali.com
Acid Base Titration Experiment Indicator Of Acid Base Titration In this article, the author has explained acid base titration, its working principle types and process of acid base titration. The reagent (titrant) is the solution with a The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. This figure shows plots of ph versus volume of. Indicator Of Acid Base Titration.
From stock.adobe.com
step of titration of acid and base or standardisation using Indicator Of Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. This figure shows plots of ph versus. Indicator Of Acid Base Titration.
From mungfali.com
Acid Base Titration Indicator Indicator Of Acid Base Titration The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. The analyte (titrand) is the solution with an unknown molarity. The synthesis of organic dyes provided many new indicators. In this article, the author has explained acid base titration, its working principle types and process of acid. Indicator Of Acid Base Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicator Of Acid Base Titration The reagent (titrant) is the solution with a The synthesis of organic dyes provided many new indicators. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of. Indicator Of Acid Base Titration.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Indicator Of Acid Base Titration How do acid base indicators work? The synthesis of organic dyes provided many new indicators. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. The reagent (titrant) is the solution with a The analyte (titrand) is the solution with an unknown molarity. This figure shows plots of ph versus. Indicator Of Acid Base Titration.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Indicator Of Acid Base Titration The synthesis of organic dyes provided many new indicators. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. The analyte (titrand) is the solution with an unknown molarity. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m. Indicator Of Acid Base Titration.
From www.biorender.com
AcidBase Titration BioRender Science Templates Indicator Of Acid Base Titration The reagent (titrant) is the solution with a The synthesis of organic dyes provided many new indicators. How do acid base indicators work? The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. In this article, the author has explained acid base titration, its working principle types. Indicator Of Acid Base Titration.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Indicator Of Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. The reagent (titrant) is the solution with a The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also. Indicator Of Acid Base Titration.
From www.shutterstock.com
Acidbase Titration Setup Phenolphthalein Indicator Vector Stock Vector Indicator Of Acid Base Titration This page assumes that you know. The synthesis of organic dyes provided many new indicators. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. The analyte (titrand) is the solution with an unknown molarity. In this article, the author has explained acid base titration, its working. Indicator Of Acid Base Titration.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Indicator Of Acid Base Titration The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. The synthesis of organic dyes provided many new indicators. This figure shows plots of ph versus volume. Indicator Of Acid Base Titration.
From chem.libretexts.org
17.3 AcidBase Indicators Chemistry LibreTexts Indicator Of Acid Base Titration In this article, the author has explained acid base titration, its working principle types and process of acid base titration. The reagent (titrant) is the solution with a How do acid base indicators work? The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. The synthesis of. Indicator Of Acid Base Titration.
From www.shutterstock.com
AcidBase Titration Setup ,Phenolphthalein Indicator Vector 253299010 Indicator Of Acid Base Titration This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. The reagent (titrant) is the solution with a The analyte (titrand) is the solution with an unknown molarity. How do acid base indicators. Indicator Of Acid Base Titration.
From www.sliderbase.com
Titration Indicator Of Acid Base Titration How do acid base indicators work? The synthesis of organic dyes provided many new indicators. The reagent (titrant) is the solution with a The analyte (titrand) is the solution with an unknown molarity. This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl). Indicator Of Acid Base Titration.
From www.vrogue.co
Titration Indicator Types Procedure Indicators vrogue.co Indicator Of Acid Base Titration The reagent (titrant) is the solution with a This figure shows plots of ph versus volume of base added for the titration of 50.0 ml of a 0.100 m solution of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. The synthesis of organic dyes provided many new indicators. The ph ranges over which two. Indicator Of Acid Base Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator Of Acid Base Titration The synthesis of organic dyes provided many new indicators. How do acid base indicators work? The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. This figure. Indicator Of Acid Base Titration.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID Indicator Of Acid Base Titration In this article, the author has explained acid base titration, its working principle types and process of acid base titration. The synthesis of organic dyes provided many new indicators. The ph ranges over which two common indicators (methyl red, pkin = 5.0, and phenolphthalein, pkin = 9.5) change color are also shown. This figure shows plots of ph versus volume. Indicator Of Acid Base Titration.