Lv Value Of Water . For example, the latent heat of vaporization of water is 540 cal/g and the latent heat of freezing of water is 80 cal/g. Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. To find this number on your own, you need to multiply the specific latent heat of the fusion of water (334 kj/kg) times the mass of. The latent heat of the fusion of 5 kg of water is 1670 kj. Specific heat of water = 1 cal/g = 4.19 kj/kg. Similarly, while ice melts, it remains at 0 °c (32. Therefore, changing a given quantity. The values of lv of water obtained were lv1 = 336 cal/g and lv2 = 338 cal/g. The units of latent heat of vaporisation are j/kg. Lv is the heat energy required to change 1kg of a liquid. Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. Latent heat of vaporisation has the symbol lv.
from www.redalyc.org
Similarly, while ice melts, it remains at 0 °c (32. The latent heat of the fusion of 5 kg of water is 1670 kj. To find this number on your own, you need to multiply the specific latent heat of the fusion of water (334 kj/kg) times the mass of. Therefore, changing a given quantity. Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. The units of latent heat of vaporisation are j/kg. Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. Lv is the heat energy required to change 1kg of a liquid. Specific heat of water = 1 cal/g = 4.19 kj/kg. The values of lv of water obtained were lv1 = 336 cal/g and lv2 = 338 cal/g.
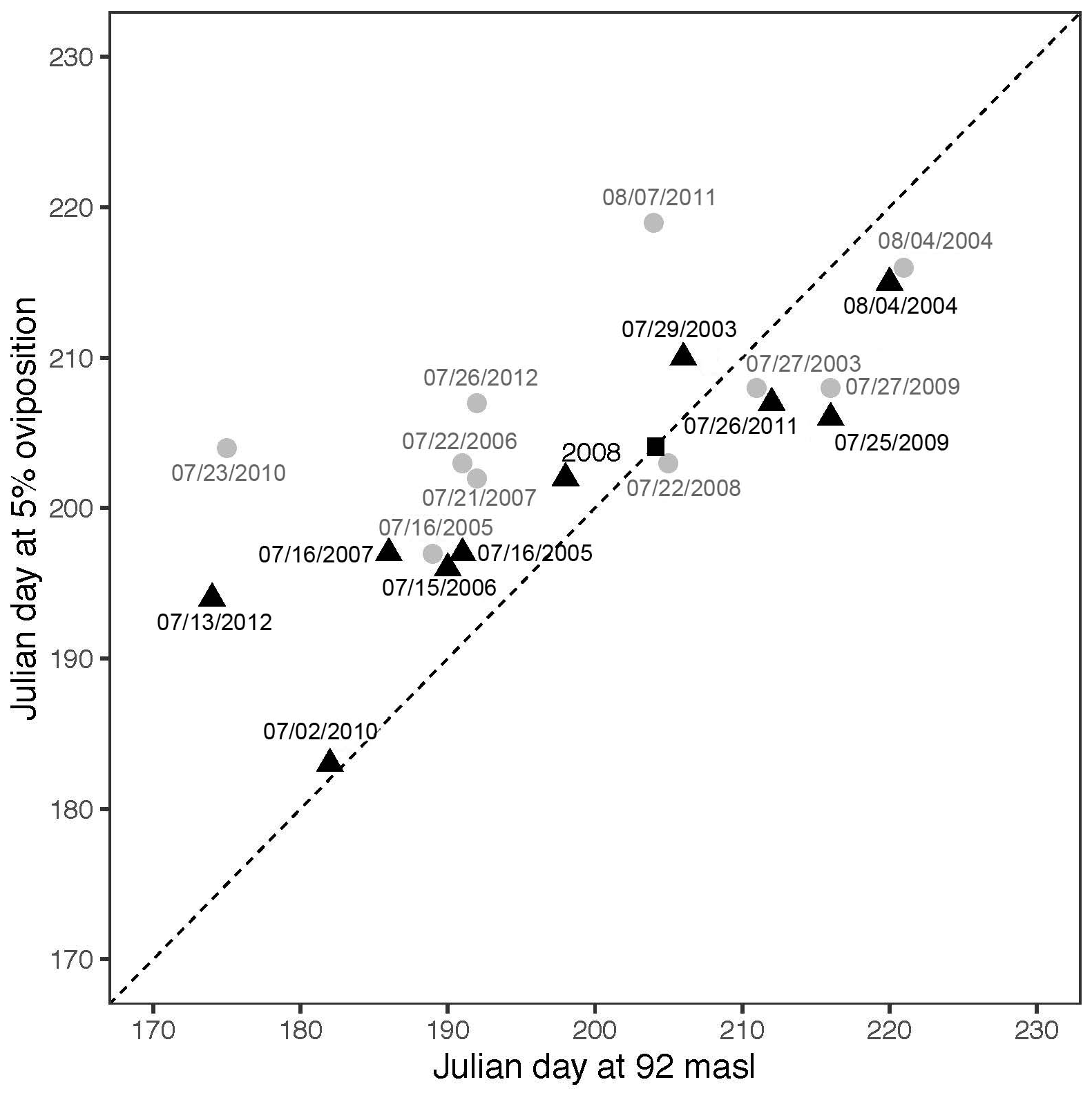
RELATIONSHIP BETWEEN OVIPOSITION OF Podocnemis unifilis AND WATER LEVEL
Lv Value Of Water Therefore, changing a given quantity. Specific heat of water = 1 cal/g = 4.19 kj/kg. The latent heat of the fusion of 5 kg of water is 1670 kj. Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. Lv is the heat energy required to change 1kg of a liquid. Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. To find this number on your own, you need to multiply the specific latent heat of the fusion of water (334 kj/kg) times the mass of. Similarly, while ice melts, it remains at 0 °c (32. For example, the latent heat of vaporization of water is 540 cal/g and the latent heat of freezing of water is 80 cal/g. The units of latent heat of vaporisation are j/kg. The values of lv of water obtained were lv1 = 336 cal/g and lv2 = 338 cal/g. Latent heat of vaporisation has the symbol lv. Therefore, changing a given quantity.
From www.tessshebaylo.com
Chemical Equation For Water Vapor Tessshebaylo Lv Value Of Water Lv is the heat energy required to change 1kg of a liquid. Similarly, while ice melts, it remains at 0 °c (32. Latent heat of vaporisation has the symbol lv. Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. For example, the latent heat of. Lv Value Of Water.
From mungfali.com
Water PH Level Chart Lv Value Of Water The latent heat of the fusion of 5 kg of water is 1670 kj. For example, the latent heat of vaporization of water is 540 cal/g and the latent heat of freezing of water is 80 cal/g. Specific heat of water = 1 cal/g = 4.19 kj/kg. Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. To. Lv Value Of Water.
From klaiiljks.blob.core.windows.net
Ph Test Of Water Pdf at Melissa Young blog Lv Value Of Water Lv is the heat energy required to change 1kg of a liquid. Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. Similarly, while ice melts, it remains at 0 °c (32. Specific heat. Lv Value Of Water.
From loedkofxe.blob.core.windows.net
Does Distilled Water Lower Ph at Robert Montez blog Lv Value Of Water The units of latent heat of vaporisation are j/kg. Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. For example, the latent heat of vaporization of water is 540 cal/g and the latent heat of freezing of water is 80 cal/g. Therefore, changing a given. Lv Value Of Water.
From locs.on.ca
Louis Vuitton Neverfull Price Increase History Timeline Literacy Lv Value Of Water For example, the latent heat of vaporization of water is 540 cal/g and the latent heat of freezing of water is 80 cal/g. Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. The values of lv of water obtained were lv1 = 336 cal/g and. Lv Value Of Water.
From mungfali.com
Ph Scale Of Water Lv Value Of Water The units of latent heat of vaporisation are j/kg. Similarly, while ice melts, it remains at 0 °c (32. Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. Latent heat of vaporisation has the symbol lv. To find this number on your own, you need to multiply the specific latent heat of the fusion of water (334. Lv Value Of Water.
From www.waterandnature.org
Lv Volumes Normal Values IUCN Water Lv Value Of Water Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. The values of lv of water obtained were lv1 = 336 cal/g and lv2 = 338 cal/g. The latent heat of the fusion of 5 kg of water is 1670 kj. Latent heat of vaporisation has. Lv Value Of Water.
From dxojtzchi.blob.core.windows.net
What Is The Normal Ph For Tap Water at James Mccord blog Lv Value Of Water Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. Lv is the heat energy required to change 1kg of a liquid. To find this number on your own, you need to multiply the specific latent heat of the fusion of water (334 kj/kg) times the mass of. Similarly, while ice melts, it remains at 0 °c (32.. Lv Value Of Water.
From www.waterandnature.org
Lv Core Values Campaign IUCN Water Lv Value Of Water Specific heat of water = 1 cal/g = 4.19 kj/kg. Similarly, while ice melts, it remains at 0 °c (32. Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. The values of lv of water obtained were lv1 = 336 cal/g and lv2 = 338. Lv Value Of Water.
From www.waterandnature.org
Lv Global Strain Normal Values IUCN Water Lv Value Of Water The latent heat of the fusion of 5 kg of water is 1670 kj. Lv is the heat energy required to change 1kg of a liquid. Therefore, changing a given quantity. For example, the latent heat of vaporization of water is 540 cal/g and the latent heat of freezing of water is 80 cal/g. The units of latent heat of. Lv Value Of Water.
From perfect-hydration.com
Drinking Water pH Explained What Should Be the Ideal pH? Perfect Lv Value Of Water Therefore, changing a given quantity. The units of latent heat of vaporisation are j/kg. Similarly, while ice melts, it remains at 0 °c (32. The latent heat of the fusion of 5 kg of water is 1670 kj. Latent heat of vaporisation has the symbol lv. To find this number on your own, you need to multiply the specific latent. Lv Value Of Water.
From nrd.kbic-nsn.gov
Lv Normal Values Natural Resource Department Lv Value Of Water Similarly, while ice melts, it remains at 0 °c (32. Lv is the heat energy required to change 1kg of a liquid. The units of latent heat of vaporisation are j/kg. The latent heat of the fusion of 5 kg of water is 1670 kj. The values of lv of water obtained were lv1 = 336 cal/g and lv2 =. Lv Value Of Water.
From www.pinterest.com
LV Water Bottle!! Lv Value Of Water Similarly, while ice melts, it remains at 0 °c (32. Latent heat of vaporisation has the symbol lv. Specific heat of water = 1 cal/g = 4.19 kj/kg. Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. Therefore, changing a given quantity. Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or. Lv Value Of Water.
From www.waterandnature.org
Normal Lv Systolic Function Ef IUCN Water Lv Value Of Water Latent heat of vaporisation has the symbol lv. To find this number on your own, you need to multiply the specific latent heat of the fusion of water (334 kj/kg) times the mass of. Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. For example, the latent heat of vaporization of water is 540 cal/g and the. Lv Value Of Water.
From gotbooks.miracosta.edu
gotbooks.miracosta.edu/oceans Lv Value Of Water To find this number on your own, you need to multiply the specific latent heat of the fusion of water (334 kj/kg) times the mass of. Therefore, changing a given quantity. Lv is the heat energy required to change 1kg of a liquid. The units of latent heat of vaporisation are j/kg. Values are usually quoted in j/mol, or kj/mol. Lv Value Of Water.
From www.waterandnature.org
Lv Wall Thickness Ase IUCN Water Lv Value Of Water Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. The units of latent heat of vaporisation are j/kg. The values of lv of water obtained were lv1 = 336 cal/g and lv2 = 338 cal/g. To find this number on your own, you need to multiply the specific latent heat of the fusion of water (334 kj/kg). Lv Value Of Water.
From exogmlhar.blob.core.windows.net
What Ph Is Good For Water at Wanda Albright blog Lv Value Of Water Latent heat of vaporisation has the symbol lv. Lv is the heat energy required to change 1kg of a liquid. To find this number on your own, you need to multiply the specific latent heat of the fusion of water (334 kj/kg) times the mass of. The values of lv of water obtained were lv1 = 336 cal/g and lv2. Lv Value Of Water.
From klabhgtws.blob.core.windows.net
Caustic Vs NonCaustic at Don Alexander blog Lv Value Of Water Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. For example, the latent heat of vaporization of water is 540 cal/g and the latent heat of freezing of water is 80 cal/g. Specific. Lv Value Of Water.
From www.researchgate.net
Global LV function of all 20 study subjects. (A) Normalized LV volume Lv Value Of Water Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. To find this number on your own, you need to multiply the specific latent heat of the fusion of water (334 kj/kg) times the mass of. The latent heat of the fusion of 5 kg of water is 1670 kj. Values are usually quoted in j/mol, or kj/mol. Lv Value Of Water.
From www.researchgate.net
Entropic level of water Download Table Lv Value Of Water The latent heat of the fusion of 5 kg of water is 1670 kj. To find this number on your own, you need to multiply the specific latent heat of the fusion of water (334 kj/kg) times the mass of. Similarly, while ice melts, it remains at 0 °c (32. For example, the latent heat of vaporization of water is. Lv Value Of Water.
From www.researchgate.net
The measured and the estimated water level values for the first water Lv Value Of Water Therefore, changing a given quantity. The latent heat of the fusion of 5 kg of water is 1670 kj. The values of lv of water obtained were lv1 = 336 cal/g and lv2 = 338 cal/g. Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like.. Lv Value Of Water.
From news.regenerativemedgroup.com
The pH of water What to know Regenerative Medical Group Lv Value Of Water Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. Latent heat of vaporisation has the symbol lv. The values of lv of water obtained were lv1 = 336 cal/g and lv2 = 338 cal/g. To find this number on your own, you need to multiply. Lv Value Of Water.
From www.researchgate.net
(PDF) Mathematical Description of the Latent Heat of Water Vaporization Lv Value Of Water Therefore, changing a given quantity. Lv is the heat energy required to change 1kg of a liquid. Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. Specific heat of water = 1 cal/g. Lv Value Of Water.
From www.bestrowaterpurifier.in
How to Check TDS Level, Ideal TDS Level of Drinking Water Lv Value Of Water Latent heat of vaporisation has the symbol lv. For example, the latent heat of vaporization of water is 540 cal/g and the latent heat of freezing of water is 80 cal/g. To find this number on your own, you need to multiply the specific latent heat of the fusion of water (334 kj/kg) times the mass of. The units of. Lv Value Of Water.
From www.redalyc.org
RELATIONSHIP BETWEEN OVIPOSITION OF Podocnemis unifilis AND WATER LEVEL Lv Value Of Water Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. The values of lv of water obtained were lv1 = 336 cal/g and lv2 = 338 cal/g. Similarly, while ice melts, it remains at 0 °c (32. The units of latent heat of vaporisation are j/kg.. Lv Value Of Water.
From www.waterandnature.org
Lv Normal Values IUCN Water Lv Value Of Water Therefore, changing a given quantity. Lv is the heat energy required to change 1kg of a liquid. Latent heat of vaporisation has the symbol lv. Similarly, while ice melts, it remains at 0 °c (32. Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. The units of latent heat of vaporisation are j/kg. For example, the latent. Lv Value Of Water.
From www.waterandnature.org
Lv Mass Normal Values Echo IUCN Water Lv Value Of Water To find this number on your own, you need to multiply the specific latent heat of the fusion of water (334 kj/kg) times the mass of. For example, the latent heat of vaporization of water is 540 cal/g and the latent heat of freezing of water is 80 cal/g. Latent heat of fusion of water = 79.5 cal/g = 333. Lv Value Of Water.
From www.slideshare.net
Water Investment Environment Lv Value Of Water The latent heat of the fusion of 5 kg of water is 1670 kj. The values of lv of water obtained were lv1 = 336 cal/g and lv2 = 338 cal/g. Latent heat of vaporisation has the symbol lv. Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. For example, the latent heat of vaporization of water. Lv Value Of Water.
From dxomiyfmg.blob.core.windows.net
Bottled Water With A Ph Of 6.0 at Jose Howard blog Lv Value Of Water Specific heat of water = 1 cal/g = 4.19 kj/kg. The units of latent heat of vaporisation are j/kg. Lv is the heat energy required to change 1kg of a liquid. To find this number on your own, you need to multiply the specific latent heat of the fusion of water (334 kj/kg) times the mass of. Latent heat of. Lv Value Of Water.
From www.researchgate.net
Observed (continuous) and predicted (dashed) water level values from Lv Value Of Water Similarly, while ice melts, it remains at 0 °c (32. Lv is the heat energy required to change 1kg of a liquid. For example, the latent heat of vaporization of water is 540 cal/g and the latent heat of freezing of water is 80 cal/g. The latent heat of the fusion of 5 kg of water is 1670 kj. Latent. Lv Value Of Water.
From renaissancecanuck.ca
Water quality and your dye pot Adventures in Arts & Sciences Lv Value Of Water The latent heat of the fusion of 5 kg of water is 1670 kj. Similarly, while ice melts, it remains at 0 °c (32. Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg.. Lv Value Of Water.
From www.researchgate.net
Measured Water Level and Estimated Water Level Values. Download Lv Value Of Water The values of lv of water obtained were lv1 = 336 cal/g and lv2 = 338 cal/g. The units of latent heat of vaporisation are j/kg. Specific heat of water = 1 cal/g = 4.19 kj/kg. For example, the latent heat of vaporization of water is 540 cal/g and the latent heat of freezing of water is 80 cal/g. Latent. Lv Value Of Water.
From netsolwater.com
What is the Normal TDS Level of Drinking Water Lv Value Of Water Similarly, while ice melts, it remains at 0 °c (32. Therefore, changing a given quantity. Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. Latent heat of fusion of water = 79.5 cal/g = 333 kj/kg. Specific heat of water = 1 cal/g = 4.19. Lv Value Of Water.
From www.slideserve.com
PPT Heat, Temperature, and Internal Energy PowerPoint Presentation Lv Value Of Water Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. Lv is the heat energy required to change 1kg of a liquid. Specific heat of water = 1 cal/g = 4.19 kj/kg. Similarly, while ice melts, it remains at 0 °c (32. Latent heat of vaporisation. Lv Value Of Water.
From www.waterandnature.org
Lv Mass Measurement Echo IUCN Water Lv Value Of Water Values are usually quoted in j/mol, or kj/mol (molar enthalpy of vaporization), although kj/kg, or j/g (specific heat of vaporization), and older units like. The values of lv of water obtained were lv1 = 336 cal/g and lv2 = 338 cal/g. Specific heat of water = 1 cal/g = 4.19 kj/kg. Lv is the heat energy required to change 1kg. Lv Value Of Water.