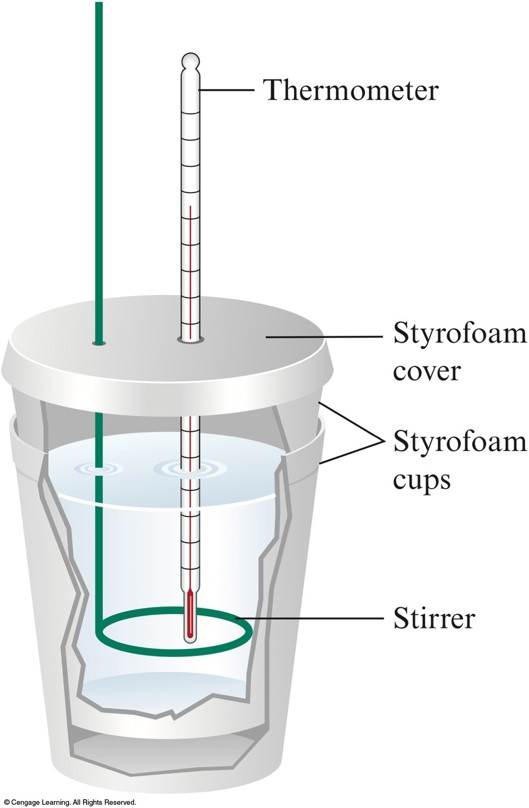
Calorimeter Are Made Up Of Which Of The Following . A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A polystyrene cup can act as a. The underlying principle is that the heat emitted by the combustion chamber causes. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. For example, when an exothermic. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The instrumentation of a calorimeter typically includes the following components: A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can.
from shaunmwilliams.com
A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A polystyrene cup can act as a. The instrumentation of a calorimeter typically includes the following components: We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. For example, when an exothermic. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. The underlying principle is that the heat emitted by the combustion chamber causes.
Chapter 5 Presentation
Calorimeter Are Made Up Of Which Of The Following The instrumentation of a calorimeter typically includes the following components: For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The underlying principle is that the heat emitted by the combustion chamber causes. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A polystyrene cup can act as a. The instrumentation of a calorimeter typically includes the following components: A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Calorimeter Are Made Up Of Which Of The Following The instrumentation of a calorimeter typically includes the following components: A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. For example, when an exothermic. A polystyrene cup can act as. Calorimeter Are Made Up Of Which Of The Following.
From exobwfkeu.blob.core.windows.net
Calorimeter Problems And Answers at Christopher Dominguez blog Calorimeter Are Made Up Of Which Of The Following For example, when an exothermic. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. The instrumentation of a calorimeter typically includes the following components: A calorimeter can be made. Calorimeter Are Made Up Of Which Of The Following.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Are Made Up Of Which Of The Following We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Calorimeter Are Made Up Of Which Of The Following.
From www.researchgate.net
The heatcapacity setup Top, schematic rendering of the new a.c Calorimeter Are Made Up Of Which Of The Following A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. For example, when an exothermic. A calorimeter is a device that measures the heat flow produced by a chemical reaction or. Calorimeter Are Made Up Of Which Of The Following.
From www.animalia-life.club
Calorimeter Diagram Calorimeter Are Made Up Of Which Of The Following The underlying principle is that the heat emitted by the combustion chamber causes. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A. Calorimeter Are Made Up Of Which Of The Following.
From www.numerade.com
SOLVED Question1(1point) Listen A 42.3 g sample of iron metal is Calorimeter Are Made Up Of Which Of The Following The instrumentation of a calorimeter typically includes the following components: A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. For example, when an exothermic. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. For example, when an exothermic. A calorimeter is a device. Calorimeter Are Made Up Of Which Of The Following.
From www.youtube.com
How to Draw a Calorimeter Step by Step Drawing Tutorial YouTube Calorimeter Are Made Up Of Which Of The Following A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A polystyrene cup can act as a. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb. Calorimeter Are Made Up Of Which Of The Following.
From chemistry.stackexchange.com
thermodynamics Heat capacity of calorimeter is negative? Chemistry Calorimeter Are Made Up Of Which Of The Following The instrumentation of a calorimeter typically includes the following components: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved. Calorimeter Are Made Up Of Which Of The Following.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimeter Are Made Up Of Which Of The Following A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter can be. Calorimeter Are Made Up Of Which Of The Following.
From shaunmwilliams.com
Chapter 5 Presentation Calorimeter Are Made Up Of Which Of The Following A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. The instrumentation of a calorimeter typically includes the following components: A polystyrene cup can act as a. The underlying principle is. Calorimeter Are Made Up Of Which Of The Following.
From glossary.periodni.com
Download calorimeter.png image from Calorimeter Are Made Up Of Which Of The Following A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. A polystyrene cup can act as a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter. Calorimeter Are Made Up Of Which Of The Following.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calorimeter Are Made Up Of Which Of The Following A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. The instrumentation of a calorimeter typically includes the following components: The underlying principle is that the heat emitted by the combustion chamber causes. A polystyrene cup can act as a. A calorimeter is a device that measures the heat flow produced by a. Calorimeter Are Made Up Of Which Of The Following.
From saylordotorg.github.io
Calorimetry Calorimeter Are Made Up Of Which Of The Following The instrumentation of a calorimeter typically includes the following components: For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter. Calorimeter Are Made Up Of Which Of The Following.
From sites.google.com
3.2.1 (e) Q=mcΔT Ellesmere OCR A level Chemistry Calorimeter Are Made Up Of Which Of The Following For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The underlying principle is that the heat emitted by the combustion chamber causes. A polystyrene cup can. Calorimeter Are Made Up Of Which Of The Following.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimeter Are Made Up Of Which Of The Following The underlying principle is that the heat emitted by the combustion chamber causes. A polystyrene cup can act as a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. For example, when an. Calorimeter Are Made Up Of Which Of The Following.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimeter Are Made Up Of Which Of The Following A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. The underlying principle is that the heat emitted by the combustion chamber causes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. A. Calorimeter Are Made Up Of Which Of The Following.
From guides.hostos.cuny.edu
Chapter 2 Energy and Matter CHE 110 Introduction to Chemistry Calorimeter Are Made Up Of Which Of The Following The underlying principle is that the heat emitted by the combustion chamber causes. A polystyrene cup can act as a. The instrumentation of a calorimeter typically includes the following components: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. We. Calorimeter Are Made Up Of Which Of The Following.
From exourbxre.blob.core.windows.net
Why Is Calorimeter Constant Important at Waldman blog Calorimeter Are Made Up Of Which Of The Following A polystyrene cup can act as a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimeter Are Made Up Of Which Of The Following.
From byjus.com
The calorimeter is commonly made up of……..because it has……. Calorimeter Are Made Up Of Which Of The Following For example, when an exothermic. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. A polystyrene cup can act as a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter can be made up of. Calorimeter Are Made Up Of Which Of The Following.
From quizzdbcerisolibf7.z13.web.core.windows.net
Heat And Calorimetry Worksheets Calorimeter Are Made Up Of Which Of The Following A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. For example, when an exothermic. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. A polystyrene cup can act as a. A calorimeter is a device used to measure. Calorimeter Are Made Up Of Which Of The Following.
From exourbxre.blob.core.windows.net
Why Is Calorimeter Constant Important at Waldman blog Calorimeter Are Made Up Of Which Of The Following A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. A. Calorimeter Are Made Up Of Which Of The Following.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Are Made Up Of Which Of The Following A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A polystyrene cup can act as a. The instrumentation of a calorimeter typically includes the following components: A calorimeter is a device. Calorimeter Are Made Up Of Which Of The Following.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimeter Are Made Up Of Which Of The Following A polystyrene cup can act as a. The underlying principle is that the heat emitted by the combustion chamber causes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. For example, when an. Calorimeter Are Made Up Of Which Of The Following.
From exohkbfgq.blob.core.windows.net
Calorimeter For Experiment at Lillian Bordner blog Calorimeter Are Made Up Of Which Of The Following We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. The instrumentation of a calorimeter typically includes the following components: A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. A calorimeter is a device used to measure the amount of. Calorimeter Are Made Up Of Which Of The Following.
From lessonlangdonprowl.z21.web.core.windows.net
Coffee Cup Calorimetry Equation Calorimeter Are Made Up Of Which Of The Following A polystyrene cup can act as a. For example, when an exothermic. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. The instrumentation of a calorimeter typically includes the following components: A calorimeter is. Calorimeter Are Made Up Of Which Of The Following.
From surfguppy.com
Enthalpy Surfguppy Chemistry made easy visual learning Calorimeter Are Made Up Of Which Of The Following The instrumentation of a calorimeter typically includes the following components: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. A calorimeter can be made up of a polystyrene. Calorimeter Are Made Up Of Which Of The Following.
From users.highland.edu
Calorimetry Calorimeter Are Made Up Of Which Of The Following For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The instrumentation of a calorimeter typically includes the following components: A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. A polystyrene cup can. Calorimeter Are Made Up Of Which Of The Following.
From chemistrytalk.org
Calorimetry ChemTalk Calorimeter Are Made Up Of Which Of The Following A polystyrene cup can act as a. For example, when an exothermic. The underlying principle is that the heat emitted by the combustion chamber causes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat. Calorimeter Are Made Up Of Which Of The Following.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Are Made Up Of Which Of The Following A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The underlying principle is that the heat emitted by the combustion chamber causes. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. A polystyrene cup can act as a. A calorimeter is. Calorimeter Are Made Up Of Which Of The Following.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Are Made Up Of Which Of The Following A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The instrumentation of a calorimeter typically includes the following components: For example, when an exothermic. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. A calorimeter can be. Calorimeter Are Made Up Of Which Of The Following.
From ar.inspiredpencil.com
Simple Bomb Calorimeter Calorimeter Are Made Up Of Which Of The Following A polystyrene cup can act as a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The underlying principle is that the heat emitted by the combustion chamber causes. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. For example, when an. Calorimeter Are Made Up Of Which Of The Following.
From www.chegg.com
Solved A certain calorimeter is made of 100 g of copper (c = Calorimeter Are Made Up Of Which Of The Following We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. The underlying principle is that the heat emitted by the combustion chamber causes. A calorimeter is a device used to measure. Calorimeter Are Made Up Of Which Of The Following.
From biobase.en.made-in-china.com
Biobase China Microprocessor Oxygen Bomb Calorimeter Bomb Calorimeter Calorimeter Are Made Up Of Which Of The Following A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. The instrumentation of a calorimeter typically includes the following components: The underlying principle is that the heat emitted by the combustion chamber causes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Calorimeter Are Made Up Of Which Of The Following.
From serc.carleton.edu
Calorimetry Calorimeter Are Made Up Of Which Of The Following We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the. For example, when an exothermic. A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can. The instrumentation of a calorimeter typically includes the following components: A calorimeter is a device that. Calorimeter Are Made Up Of Which Of The Following.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Calorimeter Are Made Up Of Which Of The Following A polystyrene cup can act as a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device that measures the heat flow produced by a chemical reaction or physical change. For example, when an exothermic. The underlying principle is that the heat emitted by the combustion. Calorimeter Are Made Up Of Which Of The Following.