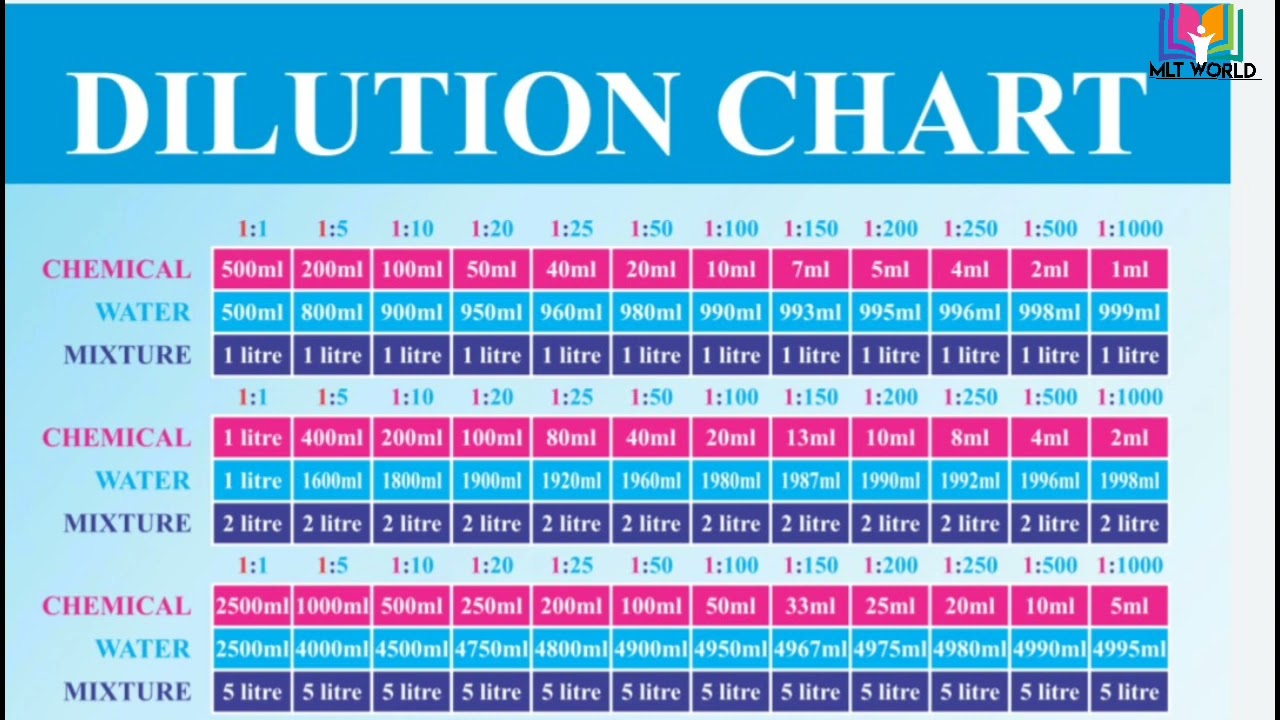
Use Dilution Factor To Calculate Concentration . You can always check your results with our dilution factor calculator or just use it in the first place. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; Meant to be used in both the teaching and research laboratory, this calculator (see below). The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution factor. The dilution factor is the inverse of the concentration factor. The formula for dilution factor involves the ratio of the stock solution volume to the dilutant volume or total volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. It works either to find the dilution. Dilution is the addition of solvent, which decreases the. Learn how to dilute and concentrate solutions.
from www.youtube.com
Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The dilution factor is the inverse of the concentration factor. State whether the concentration of a solution is directly or indirectly proportional to its volume. You can always check your results with our dilution factor calculator or just use it in the first place. Meant to be used in both the teaching and research laboratory, this calculator (see below). Learn how to dilute and concentrate solutions. The formula for dilution factor involves the ratio of the stock solution volume to the dilutant volume or total volume. It works either to find the dilution. Dilution is the addition of solvent, which decreases the. The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution factor.
Dilution Chart.Helpful video. Understand how to prepare dilutions in
Use Dilution Factor To Calculate Concentration The dilution factor is the inverse of the concentration factor. The dilution factor is the inverse of the concentration factor. The formula for dilution factor involves the ratio of the stock solution volume to the dilutant volume or total volume. State whether the concentration of a solution is directly or indirectly proportional to its volume. It works either to find the dilution. Dilution is the addition of solvent, which decreases the. You can always check your results with our dilution factor calculator or just use it in the first place. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Meant to be used in both the teaching and research laboratory, this calculator (see below). Learn how to dilute and concentrate solutions. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution factor.
From studyempathetic.z4.web.core.windows.net
Calculate Dilution Percent Use Dilution Factor To Calculate Concentration Often, a worker will need to change the concentration of a solution by changing the amount of solvent. You can always check your results with our dilution factor calculator or just use it in the first place. Meant to be used in both the teaching and research laboratory, this calculator (see below). Learn how to dilute and concentrate solutions. The. Use Dilution Factor To Calculate Concentration.
From denisseqibowen.blogspot.com
How to Calculate Dilution Factor DenisseqiBowen Use Dilution Factor To Calculate Concentration The dilution factor is the inverse of the concentration factor. Meant to be used in both the teaching and research laboratory, this calculator (see below). Learn how to dilute and concentrate solutions. Dilution is the addition of solvent, which decreases the. It works either to find the dilution. For example, if you take 1 part of a sample and add. Use Dilution Factor To Calculate Concentration.
From www.youtube.com
How to Calculate CFUSerial Dilution Microbiology Technique knowledge Use Dilution Factor To Calculate Concentration Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Meant to be used in both the teaching and research laboratory, this calculator (see below). The formula for dilution factor involves the ratio of the stock solution volume to the dilutant volume or total volume. You can always check your results with. Use Dilution Factor To Calculate Concentration.
From studyempathetic.z4.web.core.windows.net
Calculate Dilution Percent Use Dilution Factor To Calculate Concentration Meant to be used in both the teaching and research laboratory, this calculator (see below). The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution factor. The dilution factor is the inverse of the concentration factor. Learn how to dilute and concentrate solutions. You can. Use Dilution Factor To Calculate Concentration.
From denisseqibowen.blogspot.com
How to Calculate Dilution Factor DenisseqiBowen Use Dilution Factor To Calculate Concentration The formula for dilution factor involves the ratio of the stock solution volume to the dilutant volume or total volume. State whether the concentration of a solution is directly or indirectly proportional to its volume. It works either to find the dilution. The answer is basically correct (see note at the end of my answer about significant figures) but there. Use Dilution Factor To Calculate Concentration.
From dxodouqth.blob.core.windows.net
Dilution Of Control In English at Richard Blackford blog Use Dilution Factor To Calculate Concentration Meant to be used in both the teaching and research laboratory, this calculator (see below). Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. It works either to find the dilution. The formula for dilution factor involves the ratio of the stock solution volume. Use Dilution Factor To Calculate Concentration.
From loelxqvfe.blob.core.windows.net
How To Calculate The Total Dilution Factor at Jason Briggs blog Use Dilution Factor To Calculate Concentration The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution factor. You can always check your results with our dilution factor calculator or just use it in the first place. It works either to find the dilution. Learn how to dilute and concentrate solutions. Dilution. Use Dilution Factor To Calculate Concentration.
From www.youtube.com
Calculating Dilution Factor YouTube Use Dilution Factor To Calculate Concentration You can always check your results with our dilution factor calculator or just use it in the first place. The dilution factor is the inverse of the concentration factor. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Meant to be used in both the teaching and research laboratory, this calculator. Use Dilution Factor To Calculate Concentration.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration Use Dilution Factor To Calculate Concentration State whether the concentration of a solution is directly or indirectly proportional to its volume. The formula for dilution factor involves the ratio of the stock solution volume to the dilutant volume or total volume. The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution. Use Dilution Factor To Calculate Concentration.
From ar.inspiredpencil.com
Serial Dilution Diagram Use Dilution Factor To Calculate Concentration The dilution factor is the inverse of the concentration factor. Dilution is the addition of solvent, which decreases the. Learn how to dilute and concentrate solutions. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; The formula for dilution factor involves the ratio of the. Use Dilution Factor To Calculate Concentration.
From mfawriting332.web.fc2.com
How do you calculate dilution? Use Dilution Factor To Calculate Concentration The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution factor. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; You can always check your results with our dilution factor. Use Dilution Factor To Calculate Concentration.
From exytxbygt.blob.core.windows.net
Dilution Definition Literature at Mark Foster blog Use Dilution Factor To Calculate Concentration The formula for dilution factor involves the ratio of the stock solution volume to the dilutant volume or total volume. Meant to be used in both the teaching and research laboratory, this calculator (see below). State whether the concentration of a solution is directly or indirectly proportional to its volume. For example, if you take 1 part of a sample. Use Dilution Factor To Calculate Concentration.
From exozlhbpn.blob.core.windows.net
Difference Between Dilution And Reconstitution at Tawny Ellis blog Use Dilution Factor To Calculate Concentration Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of solvent, which decreases the. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; State whether the concentration of a solution is directly. Use Dilution Factor To Calculate Concentration.
From joiuugyhn.blob.core.windows.net
Dilution Calculator Concentration at Bernard Butler blog Use Dilution Factor To Calculate Concentration You can always check your results with our dilution factor calculator or just use it in the first place. Learn how to dilute and concentrate solutions. Meant to be used in both the teaching and research laboratory, this calculator (see below). It works either to find the dilution. State whether the concentration of a solution is directly or indirectly proportional. Use Dilution Factor To Calculate Concentration.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria Use Dilution Factor To Calculate Concentration Learn how to dilute and concentrate solutions. You can always check your results with our dilution factor calculator or just use it in the first place. It works either to find the dilution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The answer is basically correct (see note at the. Use Dilution Factor To Calculate Concentration.
From blog.naver.com
Antibody Titer 정량을 위한 rProteinAHPLC 네이버 블로그 Use Dilution Factor To Calculate Concentration The dilution factor is the inverse of the concentration factor. You can always check your results with our dilution factor calculator or just use it in the first place. Meant to be used in both the teaching and research laboratory, this calculator (see below). Learn how to dilute and concentrate solutions. The formula for dilution factor involves the ratio of. Use Dilution Factor To Calculate Concentration.
From www.integra-biosciences.com
How to do serial dilutions (including calculations) INTEGRA Use Dilution Factor To Calculate Concentration The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution factor. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The dilution factor is the inverse of the concentration factor. State whether the concentration of a. Use Dilution Factor To Calculate Concentration.
From sciencesavers.info
System, Calculator, Methodology, Makes use of, Examples sciencesavers Use Dilution Factor To Calculate Concentration Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution factor. For example, if you take 1 part of a sample and add 9 parts of water. Use Dilution Factor To Calculate Concentration.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Use Dilution Factor To Calculate Concentration Dilution is the addition of solvent, which decreases the. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; The formula for dilution factor involves the ratio of the stock solution volume to the dilutant volume or total volume. State whether the concentration of a solution. Use Dilution Factor To Calculate Concentration.
From loelxqvfe.blob.core.windows.net
How To Calculate The Total Dilution Factor at Jason Briggs blog Use Dilution Factor To Calculate Concentration Learn how to dilute and concentrate solutions. It works either to find the dilution. Meant to be used in both the teaching and research laboratory, this calculator (see below). The formula for dilution factor involves the ratio of the stock solution volume to the dilutant volume or total volume. The dilution factor is the inverse of the concentration factor. You. Use Dilution Factor To Calculate Concentration.
From www.youtube.com
How to Calculate Dilution Factor YouTube Use Dilution Factor To Calculate Concentration Meant to be used in both the teaching and research laboratory, this calculator (see below). The formula for dilution factor involves the ratio of the stock solution volume to the dilutant volume or total volume. State whether the concentration of a solution is directly or indirectly proportional to its volume. It works either to find the dilution. For example, if. Use Dilution Factor To Calculate Concentration.
From socratic.org
How can I calculate the dilution factor using concentration? Socratic Use Dilution Factor To Calculate Concentration Meant to be used in both the teaching and research laboratory, this calculator (see below). Often, a worker will need to change the concentration of a solution by changing the amount of solvent. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; You can always. Use Dilution Factor To Calculate Concentration.
From www.hemocytometer.org
Dilution factor calculator • Hemocytometer Use Dilution Factor To Calculate Concentration Learn how to dilute and concentrate solutions. Dilution is the addition of solvent, which decreases the. The formula for dilution factor involves the ratio of the stock solution volume to the dilutant volume or total volume. You can always check your results with our dilution factor calculator or just use it in the first place. For example, if you take. Use Dilution Factor To Calculate Concentration.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Use Dilution Factor To Calculate Concentration The formula for dilution factor involves the ratio of the stock solution volume to the dilutant volume or total volume. Dilution is the addition of solvent, which decreases the. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration. Use Dilution Factor To Calculate Concentration.
From www.youtube.com
Dilution concentration calculation YouTube Use Dilution Factor To Calculate Concentration Dilution is the addition of solvent, which decreases the. The dilution factor is the inverse of the concentration factor. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10. Use Dilution Factor To Calculate Concentration.
From joiuugyhn.blob.core.windows.net
Dilution Calculator Concentration at Bernard Butler blog Use Dilution Factor To Calculate Concentration Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. The answer is basically correct (see note at the end of my answer about significant figures) but there is a. Use Dilution Factor To Calculate Concentration.
From dxobdxqob.blob.core.windows.net
Purple Power Dilution Ratio at Martin Tully blog Use Dilution Factor To Calculate Concentration You can always check your results with our dilution factor calculator or just use it in the first place. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of. Use Dilution Factor To Calculate Concentration.
From studylib.net
Dilution Ratios Table Use Dilution Factor To Calculate Concentration Often, a worker will need to change the concentration of a solution by changing the amount of solvent. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; State whether the concentration of a solution is directly or indirectly proportional to its volume. You can always. Use Dilution Factor To Calculate Concentration.
From ceysjldb.blob.core.windows.net
How To Graph Serial Dilutions at Linda Pike blog Use Dilution Factor To Calculate Concentration It works either to find the dilution. Learn how to dilute and concentrate solutions. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; Often, a worker will need to change the concentration of a solution by changing the amount of solvent. You can always check. Use Dilution Factor To Calculate Concentration.
From joiuugyhn.blob.core.windows.net
Dilution Calculator Concentration at Bernard Butler blog Use Dilution Factor To Calculate Concentration It works either to find the dilution. You can always check your results with our dilution factor calculator or just use it in the first place. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; Often, a worker will need to change the concentration of. Use Dilution Factor To Calculate Concentration.
From www.youtube.com
Dilution determining final concentration (example) YouTube Use Dilution Factor To Calculate Concentration Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Meant to be used in both the teaching and research laboratory, this calculator (see below). The dilution factor is the inverse of the concentration factor. Learn how to dilute and concentrate solutions. It works either to find the dilution. State whether the. Use Dilution Factor To Calculate Concentration.
From lefasamtucker.blogspot.com
How to Calculate Concentration of a Solution Sam Tucker Use Dilution Factor To Calculate Concentration You can always check your results with our dilution factor calculator or just use it in the first place. It works either to find the dilution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The answer is basically correct (see note at the end of my answer about significant figures). Use Dilution Factor To Calculate Concentration.
From dxopazssz.blob.core.windows.net
Dilution Calculator From Mass at Blanca Norton blog Use Dilution Factor To Calculate Concentration State whether the concentration of a solution is directly or indirectly proportional to its volume. You can always check your results with our dilution factor calculator or just use it in the first place. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The. Use Dilution Factor To Calculate Concentration.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Use Dilution Factor To Calculate Concentration The formula for dilution factor involves the ratio of the stock solution volume to the dilutant volume or total volume. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the. Learn how to dilute and concentrate solutions. The answer is basically correct (see note at the. Use Dilution Factor To Calculate Concentration.
From www.youtube.com
Dilution Calculation Practice YouTube Use Dilution Factor To Calculate Concentration For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The formula for dilution factor involves the ratio of the stock solution volume to the dilutant volume or. Use Dilution Factor To Calculate Concentration.