Dilution Error Calculation . how to perform serial dilutions with high accuracy. Serial dilutions crucially depend on excellent liquid handling. ” these are very different. dilutions can be performed as “ diluted to ” or “ diluted to a final volume of ” or as “ diluted with. Calculate the minimum diluent volume per step: state whether the concentration of a solution is directly or indirectly proportional to its volume. 50 μl per well * 2 for duplicates = 100 μl minimum. the ‘best way’ would be to have minimal dilution error (i.e., smallest measurement uncertainty commensurate. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. dilution = volume or mass of sample / total volume or mass of (sample + diluent) dilution factor = total v of (sample + diluent) / v of. From the standard deviation of. four steps for determining the uncertainty of a measurement step 1.
from www.youtube.com
50 μl per well * 2 for duplicates = 100 μl minimum. how to perform serial dilutions with high accuracy. Serial dilutions crucially depend on excellent liquid handling. four steps for determining the uncertainty of a measurement step 1. ” these are very different. dilution = volume or mass of sample / total volume or mass of (sample + diluent) dilution factor = total v of (sample + diluent) / v of. dilutions can be performed as “ diluted to ” or “ diluted to a final volume of ” or as “ diluted with. Calculate the minimum diluent volume per step: the ‘best way’ would be to have minimal dilution error (i.e., smallest measurement uncertainty commensurate. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as.
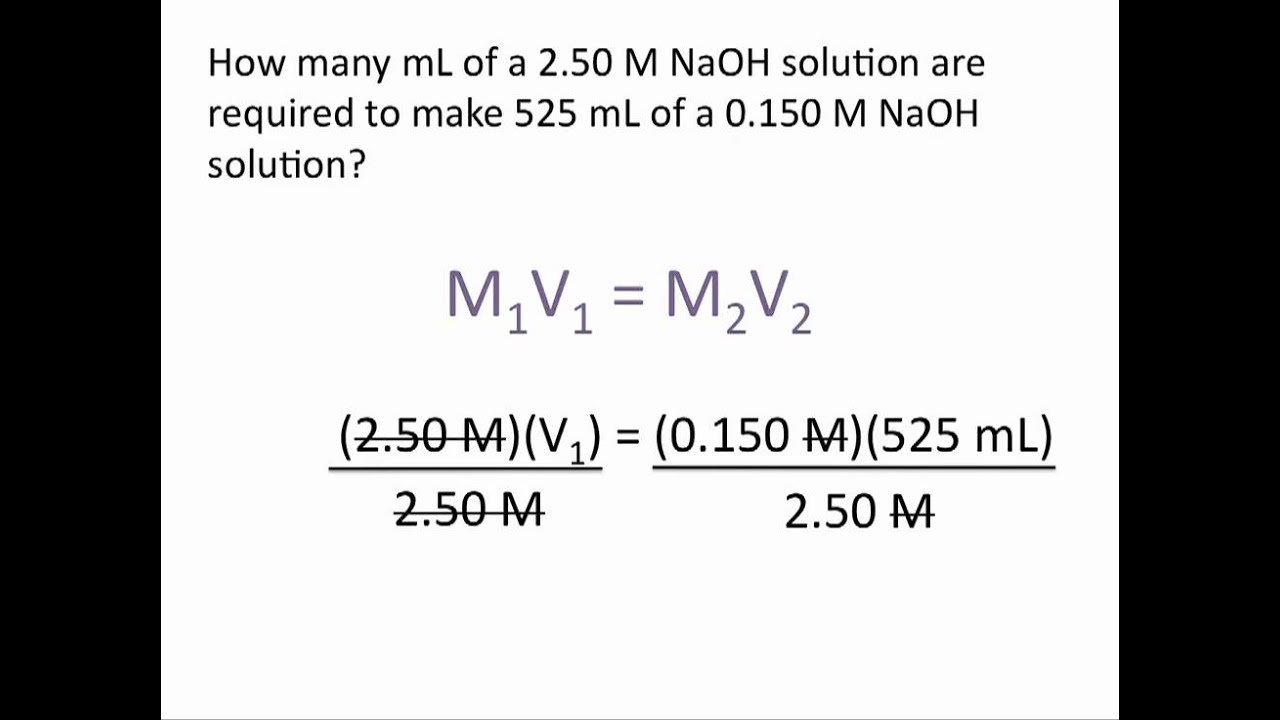
Dilution Problems Chemistry Tutorial YouTube
Dilution Error Calculation four steps for determining the uncertainty of a measurement step 1. Calculate the minimum diluent volume per step: 50 μl per well * 2 for duplicates = 100 μl minimum. how to perform serial dilutions with high accuracy. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. four steps for determining the uncertainty of a measurement step 1. dilution = volume or mass of sample / total volume or mass of (sample + diluent) dilution factor = total v of (sample + diluent) / v of. the ‘best way’ would be to have minimal dilution error (i.e., smallest measurement uncertainty commensurate. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilutions can be performed as “ diluted to ” or “ diluted to a final volume of ” or as “ diluted with. From the standard deviation of. Serial dilutions crucially depend on excellent liquid handling. ” these are very different.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Dilution Error Calculation ” these are very different. how to perform serial dilutions with high accuracy. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. From the standard deviation of. 50 μl per well * 2 for duplicates = 100 μl minimum. the ‘best way’. Dilution Error Calculation.
From dxlwoimeeco.blob.core.windows.net
Dilution Calculation Formula at Bertha Mohr blog Dilution Error Calculation Serial dilutions crucially depend on excellent liquid handling. From the standard deviation of. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. four steps for determining the uncertainty of a measurement step 1. how to perform serial dilutions with high accuracy. . Dilution Error Calculation.
From dhsdwppleco.blob.core.windows.net
Dilution Calculator Lab Hacks at Earl Curry blog Dilution Error Calculation dilutions can be performed as “ diluted to ” or “ diluted to a final volume of ” or as “ diluted with. Calculate the minimum diluent volume per step: how to perform serial dilutions with high accuracy. the ‘best way’ would be to have minimal dilution error (i.e., smallest measurement uncertainty commensurate. Serial dilutions crucially depend. Dilution Error Calculation.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Error Calculation how to perform serial dilutions with high accuracy. dilutions can be performed as “ diluted to ” or “ diluted to a final volume of ” or as “ diluted with. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. the. Dilution Error Calculation.
From windowsdiary.com
Troubleshooting Tips for Propagating Serial Dilution Errors Windows Diary Dilution Error Calculation this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. 50 μl per well * 2 for duplicates = 100 μl minimum. four steps for determining the uncertainty of a measurement step 1. ” these are very different. Calculate the minimum diluent volume per. Dilution Error Calculation.
From cehvgbxe.blob.core.windows.net
Dilution Calculations Nursing at Efren Plumley blog Dilution Error Calculation From the standard deviation of. dilution = volume or mass of sample / total volume or mass of (sample + diluent) dilution factor = total v of (sample + diluent) / v of. state whether the concentration of a solution is directly or indirectly proportional to its volume. the ‘best way’ would be to have minimal dilution. Dilution Error Calculation.
From www.youtube.com
Microbiology Serial Dilution calculation YouTube Dilution Error Calculation Serial dilutions crucially depend on excellent liquid handling. the ‘best way’ would be to have minimal dilution error (i.e., smallest measurement uncertainty commensurate. ” these are very different. 50 μl per well * 2 for duplicates = 100 μl minimum. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform. Dilution Error Calculation.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID Dilution Error Calculation this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. four steps for determining the uncertainty of a measurement step 1. Serial dilutions crucially depend on excellent liquid handling. the ‘best way’ would be to have minimal dilution error (i.e., smallest measurement uncertainty. Dilution Error Calculation.
From www.researchgate.net
Total Number of Dilutions and Dilution Errors by Drug Download Table Dilution Error Calculation 50 μl per well * 2 for duplicates = 100 μl minimum. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. the ‘best way’ would be to have minimal dilution error (i.e., smallest measurement uncertainty commensurate. state whether the concentration of a. Dilution Error Calculation.
From dxlwoimeeco.blob.core.windows.net
Dilution Calculation Formula at Bertha Mohr blog Dilution Error Calculation how to perform serial dilutions with high accuracy. From the standard deviation of. Calculate the minimum diluent volume per step: Serial dilutions crucially depend on excellent liquid handling. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. the ‘best way’ would be. Dilution Error Calculation.
From cevxwmet.blob.core.windows.net
Dilution Series Calculation at Darren Burgess blog Dilution Error Calculation ” these are very different. Calculate the minimum diluent volume per step: four steps for determining the uncertainty of a measurement step 1. state whether the concentration of a solution is directly or indirectly proportional to its volume. how to perform serial dilutions with high accuracy. 50 μl per well * 2 for duplicates = 100 μl. Dilution Error Calculation.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution Error Calculation state whether the concentration of a solution is directly or indirectly proportional to its volume. four steps for determining the uncertainty of a measurement step 1. dilutions can be performed as “ diluted to ” or “ diluted to a final volume of ” or as “ diluted with. Calculate the minimum diluent volume per step: Serial. Dilution Error Calculation.
From www.slideserve.com
PPT Statistical and practical challenges in estimating flows in Dilution Error Calculation Calculate the minimum diluent volume per step: dilution = volume or mass of sample / total volume or mass of (sample + diluent) dilution factor = total v of (sample + diluent) / v of. how to perform serial dilutions with high accuracy. four steps for determining the uncertainty of a measurement step 1. 50 μl per. Dilution Error Calculation.
From www.researchgate.net
(PDF) Calculation error following sample dilution a proposal for Dilution Error Calculation how to perform serial dilutions with high accuracy. dilution = volume or mass of sample / total volume or mass of (sample + diluent) dilution factor = total v of (sample + diluent) / v of. 50 μl per well * 2 for duplicates = 100 μl minimum. dilutions can be performed as “ diluted to ”. Dilution Error Calculation.
From www.educba.com
Dilution Formula Calculator (Examples with Excel Template) Dilution Error Calculation how to perform serial dilutions with high accuracy. ” these are very different. Calculate the minimum diluent volume per step: four steps for determining the uncertainty of a measurement step 1. 50 μl per well * 2 for duplicates = 100 μl minimum. state whether the concentration of a solution is directly or indirectly proportional to its. Dilution Error Calculation.
From www.youtube.com
Calculate dilution factor and concentration when diluting liquid and Dilution Error Calculation four steps for determining the uncertainty of a measurement step 1. dilutions can be performed as “ diluted to ” or “ diluted to a final volume of ” or as “ diluted with. state whether the concentration of a solution is directly or indirectly proportional to its volume. Serial dilutions crucially depend on excellent liquid handling.. Dilution Error Calculation.
From www.youtube.com
Dilution Practice Problems & Example Problems YouTube Dilution Error Calculation ” these are very different. the ‘best way’ would be to have minimal dilution error (i.e., smallest measurement uncertainty commensurate. dilutions can be performed as “ diluted to ” or “ diluted to a final volume of ” or as “ diluted with. dilution = volume or mass of sample / total volume or mass of (sample. Dilution Error Calculation.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Error Calculation this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. From the standard deviation of. four steps for determining the uncertainty of a measurement step 1. Serial dilutions crucially depend on excellent liquid handling. 50 μl per well * 2 for duplicates = 100. Dilution Error Calculation.
From exookhlfi.blob.core.windows.net
Dilution Factor Calculation In Hplc at John Comer blog Dilution Error Calculation four steps for determining the uncertainty of a measurement step 1. Calculate the minimum diluent volume per step: this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. From the standard deviation of. state whether the concentration of a solution is directly or. Dilution Error Calculation.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Error Calculation this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. the ‘best way’ would be to have minimal dilution error (i.e., smallest measurement uncertainty commensurate. how to perform serial dilutions with high accuracy. From the standard deviation of. dilution = volume or. Dilution Error Calculation.
From www.youtube.com
Paracetamol Assay as per IP specific absorbance dilutions back Dilution Error Calculation Calculate the minimum diluent volume per step: From the standard deviation of. how to perform serial dilutions with high accuracy. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. the ‘best way’ would be to have minimal dilution error (i.e., smallest measurement. Dilution Error Calculation.
From www.chegg.com
Solved a) Calculate the dilution factor of each dilution, Dilution Error Calculation 50 μl per well * 2 for duplicates = 100 μl minimum. the ‘best way’ would be to have minimal dilution error (i.e., smallest measurement uncertainty commensurate. dilution = volume or mass of sample / total volume or mass of (sample + diluent) dilution factor = total v of (sample + diluent) / v of. From the standard. Dilution Error Calculation.
From www.youtube.com
Solution dilution calculations YouTube Dilution Error Calculation dilution = volume or mass of sample / total volume or mass of (sample + diluent) dilution factor = total v of (sample + diluent) / v of. 50 μl per well * 2 for duplicates = 100 μl minimum. the ‘best way’ would be to have minimal dilution error (i.e., smallest measurement uncertainty commensurate. this article. Dilution Error Calculation.
From criticalthinking.cloud
dilution concentration calculation problems Dilution Error Calculation this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. dilutions can be performed as “ diluted to ” or “ diluted to a final volume of ” or as “ diluted with. 50 μl per well * 2 for duplicates = 100 μl. Dilution Error Calculation.
From dxlwoimeeco.blob.core.windows.net
Dilution Calculation Formula at Bertha Mohr blog Dilution Error Calculation how to perform serial dilutions with high accuracy. From the standard deviation of. Calculate the minimum diluent volume per step: dilutions can be performed as “ diluted to ” or “ diluted to a final volume of ” or as “ diluted with. ” these are very different. 50 μl per well * 2 for duplicates = 100. Dilution Error Calculation.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Error Calculation ” these are very different. Serial dilutions crucially depend on excellent liquid handling. From the standard deviation of. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. state whether the concentration of a solution is directly or indirectly proportional to its volume. Calculate. Dilution Error Calculation.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Error Calculation Serial dilutions crucially depend on excellent liquid handling. dilution = volume or mass of sample / total volume or mass of (sample + diluent) dilution factor = total v of (sample + diluent) / v of. four steps for determining the uncertainty of a measurement step 1. Calculate the minimum diluent volume per step: From the standard deviation. Dilution Error Calculation.
From cevxwmet.blob.core.windows.net
Dilution Series Calculation at Darren Burgess blog Dilution Error Calculation dilutions can be performed as “ diluted to ” or “ diluted to a final volume of ” or as “ diluted with. From the standard deviation of. dilution = volume or mass of sample / total volume or mass of (sample + diluent) dilution factor = total v of (sample + diluent) / v of. ” these. Dilution Error Calculation.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Error Calculation how to perform serial dilutions with high accuracy. Calculate the minimum diluent volume per step: this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. dilution = volume or mass of sample / total volume or mass of (sample + diluent) dilution factor. Dilution Error Calculation.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution Error Calculation Calculate the minimum diluent volume per step: dilutions can be performed as “ diluted to ” or “ diluted to a final volume of ” or as “ diluted with. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. ” these are very. Dilution Error Calculation.
From cfmuvi.jimdo.com
Simple Serial Dilution Calculation cfmuvi Dilution Error Calculation this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. how to perform serial dilutions with high accuracy. dilution = volume or mass of sample / total volume or mass of (sample + diluent) dilution factor = total v of (sample + diluent). Dilution Error Calculation.
From www.youtube.com
How to Calculate Dilution Factor YouTube Dilution Error Calculation dilution = volume or mass of sample / total volume or mass of (sample + diluent) dilution factor = total v of (sample + diluent) / v of. Serial dilutions crucially depend on excellent liquid handling. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as. Dilution Error Calculation.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Error Calculation the ‘best way’ would be to have minimal dilution error (i.e., smallest measurement uncertainty commensurate. ” these are very different. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilutions can be performed as “ diluted to ” or “ diluted to a final volume of ” or as “ diluted. Dilution Error Calculation.
From contactlasopa661.weebly.com
Serial dilution sources of error in measurement physics contactlasopa Dilution Error Calculation this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as. the ‘best way’ would be to have minimal dilution error (i.e., smallest measurement uncertainty commensurate. Serial dilutions crucially depend on excellent liquid handling. dilution = volume or mass of sample / total volume. Dilution Error Calculation.
From newact446.weebly.com
Serial Dilution Calculation Examples newact Dilution Error Calculation how to perform serial dilutions with high accuracy. Serial dilutions crucially depend on excellent liquid handling. 50 μl per well * 2 for duplicates = 100 μl minimum. Calculate the minimum diluent volume per step: From the standard deviation of. ” these are very different. dilution = volume or mass of sample / total volume or mass of. Dilution Error Calculation.