Aluminum Sulfate The Correct Formula . In this video we'll write the correct formula for aluminum sulfate, al(so4)3.to write the formula for aluminum sulfate we'll use the. Aluminum sulfate can be prepared using a simple chemical reaction where aluminum hydroxide reacts with sulfuric acid. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Sulfate is a polyatomic ion with formula (so4)2−. The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. Aluminum sulfate has the chemical formula al2(so4)3. The chemical equation for this reaction is: It is a white crystalline solid in its anhydrous. The solution that results is. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. The formula can be derived by identifying the elements present in the. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. Aluminum is a group 3a metal which forms a 3 + cation.
from www.numerade.com
The solution that results is. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Aluminum sulfate can be prepared using a simple chemical reaction where aluminum hydroxide reacts with sulfuric acid. Aluminum sulfate has the chemical formula al2(so4)3. In this video we'll write the correct formula for aluminum sulfate, al(so4)3.to write the formula for aluminum sulfate we'll use the. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o The formula can be derived by identifying the elements present in the. The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. The chemical equation for this reaction is: The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}.
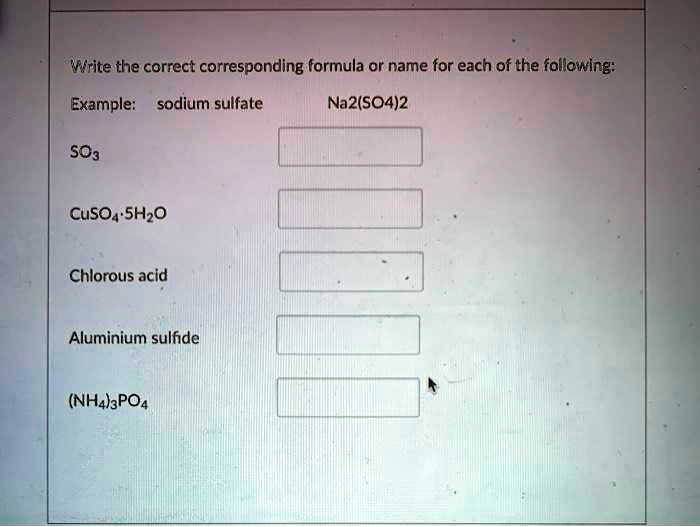
SOLVEDWrite the correct corresponding formula or name for each of the
Aluminum Sulfate The Correct Formula The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o Aluminum sulfate can be prepared using a simple chemical reaction where aluminum hydroxide reacts with sulfuric acid. The chemical equation for this reaction is: Sulfate is a polyatomic ion with formula (so4)2−. It is a white crystalline solid in its anhydrous. The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. Aluminum sulfate has the chemical formula al2(so4)3. In this video we'll write the correct formula for aluminum sulfate, al(so4)3.to write the formula for aluminum sulfate we'll use the. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. Aluminum is a group 3a metal which forms a 3 + cation. The formula can be derived by identifying the elements present in the. The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. The solution that results is.
From www.chegg.com
Solved Give the correct formula for aluminum sulfate. O Aluminum Sulfate The Correct Formula The formula can be derived by identifying the elements present in the. The chemical equation for this reaction is: The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. It is a white crystalline solid in its anhydrous. Aluminum sulfate has the chemical formula. Aluminum Sulfate The Correct Formula.
From aydin-blognorton.blogspot.com
What Is the Formula for Aluminum Sulfite Aluminum Sulfate The Correct Formula 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o Sulfate is a polyatomic ion with formula (so4)2−. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Aluminum is a group 3a metal which forms a 3 + cation. Aluminum sulfate has the chemical formula al2(so4)3. The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. The. Aluminum Sulfate The Correct Formula.
From www.museoinclusivo.com
Exploring Aluminum Sulfate Formula Uses, Chemical Properties, and Aluminum Sulfate The Correct Formula 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. It is a white crystalline solid in its anhydrous.. Aluminum Sulfate The Correct Formula.
From www.fishersci.ca
Aluminium sulfate, 99.999, (trace metal basis), extra pure, ACROS Aluminum Sulfate The Correct Formula The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. Sulfate is a polyatomic ion with formula (so4)2−. Aluminum sulfate has the chemical formula al2(so4)3. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. The chemical equation for. Aluminum Sulfate The Correct Formula.
From www.meritnation.com
Write down the formulae of aluminium sulphate and ethanol Ans Aluminum Sulfate The Correct Formula It is a white crystalline solid in its anhydrous. Aluminum is a group 3a metal which forms a 3 + cation. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. Sulfate is a polyatomic ion with formula (so4)2−. The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. The solution. Aluminum Sulfate The Correct Formula.
From www.youtube.com
How to Write the Formula for Aluminum sulfate YouTube Aluminum Sulfate The Correct Formula Aluminum is a group 3a metal which forms a 3 + cation. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o The formula can be derived by identifying the elements present in the. Aluminum sulfate has the chemical formula al2(so4)3. The solution that results is. Sulfate is a polyatomic ion with formula (so4)2−. Aluminium sulphate is formed by reacting with the correct. Aluminum Sulfate The Correct Formula.
From www.doubtnut.com
The correct formula of aluminium sulphate is AlSO(4). Aluminum Sulfate The Correct Formula The solution that results is. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Aluminum is a group 3a metal which forms a 3 + cation. Aluminum sulfate can be prepared using a simple chemical reaction where aluminum hydroxide reacts with sulfuric acid. Aluminum sulfate, often referred to as alum, is. Aluminum Sulfate The Correct Formula.
From www.pinterest.com
Aluminium sulfate Al2(SO4)3 Molecular Geometry Hybridization Aluminum Sulfate The Correct Formula The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o It is a white crystalline solid in its anhydrous. Aluminum is a group 3a metal which forms a 3 + cation. Sulfate is a polyatomic ion with formula (so4)2−. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so. Aluminum Sulfate The Correct Formula.
From www.youtube.com
what is the percent composition of aluminum sulfate? YouTube Aluminum Sulfate The Correct Formula Aluminum is a group 3a metal which forms a 3 + cation. The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. Sulfate is a polyatomic ion with formula (so4)2−. The chemical equation for this reaction is: Aluminum sulfate, often referred to as alum, is a chemical compound. Aluminum Sulfate The Correct Formula.
From molekula.com
Purchase Aluminum sulfate hexadecahydrate [16828118] online • Catalog Aluminum Sulfate The Correct Formula Sulfate is a polyatomic ion with formula (so4)2−. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o The chemical equation for this reaction is: The solution that results is. The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. Aluminum sulfate can be prepared using a simple chemical reaction where aluminum hydroxide reacts with sulfuric acid. It is a white crystalline solid in its anhydrous.. Aluminum Sulfate The Correct Formula.
From www.slideserve.com
PPT Percentage Composition PowerPoint Presentation, free download Aluminum Sulfate The Correct Formula Aluminum sulfate has the chemical formula al2(so4)3. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Aluminum is a group 3a metal which forms a 3 + cation. In this video we'll write the correct formula for aluminum sulfate, al(so4)3.to write the formula for aluminum sulfate we'll use the. The chemical. Aluminum Sulfate The Correct Formula.
From www.youtube.com
What is the chemical formula of Aluminium sulphate ? // chemical name Aluminum Sulfate The Correct Formula Sulfate is a polyatomic ion with formula (so4)2−. The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. The chemical equation for this reaction is: The solution that results is. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Aluminum is a group 3a metal which forms a 3 + cation. 2al(oh)₃ + 3h₂so₄. Aluminum Sulfate The Correct Formula.
From hxewxvhin.blob.core.windows.net
How To Aluminum Sulfate Formula at Caitlin Hardin blog Aluminum Sulfate The Correct Formula The solution that results is. Aluminum is a group 3a metal which forms a 3 + cation. It is a white crystalline solid in its anhydrous. Aluminum sulfate can be prepared using a simple chemical reaction where aluminum hydroxide reacts with sulfuric acid. The formula can be derived by identifying the elements present in the. Aluminum sulfate has the chemical. Aluminum Sulfate The Correct Formula.
From www.dreamstime.com
3D Image of Aluminum Sulfate Skeletal Formula Stock Illustration Aluminum Sulfate The Correct Formula The chemical equation for this reaction is: The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. Aluminum is a group 3a metal which forms a 3 + cation. The formula can be derived by identifying the elements present in the. Sulfate is a polyatomic ion with formula (so4)2−. The solution that results is. It is a white crystalline solid in its anhydrous.. Aluminum Sulfate The Correct Formula.
From www.slideserve.com
PPT Calculating Chemical Equations PowerPoint Presentation, free Aluminum Sulfate The Correct Formula The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. Sulfate is a polyatomic ion with formula (so4)2−. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o The chemical equation for this reaction is: Aluminum sulfate can be prepared using a simple chemical reaction where aluminum hydroxide reacts with sulfuric acid. In this video we'll write the correct formula for aluminum sulfate, al(so4)3.to write the. Aluminum Sulfate The Correct Formula.
From www.youtube.com
How to write the formula for aluminum sulfate YouTube Aluminum Sulfate The Correct Formula Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. It is a white crystalline solid in its anhydrous. In this video we'll write the correct formula for aluminum sulfate, al(so4)3.to write the formula for aluminum sulfate we'll use the. The chemical formula shows that a single aluminum sulfate molecule is composed. Aluminum Sulfate The Correct Formula.
From brainly.in
molecular formula of aluminium sulphate with steps Brainly.in Aluminum Sulfate The Correct Formula In this video we'll write the correct formula for aluminum sulfate, al(so4)3.to write the formula for aluminum sulfate we'll use the. The solution that results is. The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. Aluminum sulfate has the chemical formula al2(so4)3. The formula can be derived. Aluminum Sulfate The Correct Formula.
From clairekruwmunoz.blogspot.com
Give the Correct Formula for Aluminum Sulfate. ClairekruwMunoz Aluminum Sulfate The Correct Formula In this video we'll write the correct formula for aluminum sulfate, al(so4)3.to write the formula for aluminum sulfate we'll use the. Aluminum is a group 3a metal which forms a 3 + cation. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. It is a white crystalline solid in its anhydrous. The solution that results. Aluminum Sulfate The Correct Formula.
From www.numerade.com
SOLVEDWrite the correct corresponding formula or name for each of the Aluminum Sulfate The Correct Formula Aluminum sulfate has the chemical formula al2(so4)3. The solution that results is. It is a white crystalline solid in its anhydrous. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o Sulfate is a polyatomic ion with formula (so4)2−. The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. Aluminum sulfate can be prepared using a simple chemical. Aluminum Sulfate The Correct Formula.
From www.youtube.com
How to write chemical formula of Aluminium SulfateAluminium Sulfate Aluminum Sulfate The Correct Formula Aluminum sulfate can be prepared using a simple chemical reaction where aluminum hydroxide reacts with sulfuric acid. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. The solution that results is. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o The chemical formula shows that a single aluminum sulfate molecule is composed of. Aluminum Sulfate The Correct Formula.
From slideplayer.com
Chemical Equations & Reactions ppt download Aluminum Sulfate The Correct Formula The formula can be derived by identifying the elements present in the. In this video we'll write the correct formula for aluminum sulfate, al(so4)3.to write the formula for aluminum sulfate we'll use the. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o It is. Aluminum Sulfate The Correct Formula.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation ID2031508 Aluminum Sulfate The Correct Formula Aluminum sulfate has the chemical formula al2(so4)3. Sulfate is a polyatomic ion with formula (so4)2−. It is a white crystalline solid in its anhydrous. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Aluminum sulfate can be prepared using a simple chemical reaction where aluminum hydroxide reacts with sulfuric acid. The. Aluminum Sulfate The Correct Formula.
From www.museoinclusivo.com
What is the Chemical Formula for Aluminum Sulfate? Aluminum Profile Blog Aluminum Sulfate The Correct Formula The solution that results is. It is a white crystalline solid in its anhydrous. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. In this video we'll write the correct formula for aluminum sulfate, al(so4)3.to write the formula for aluminum sulfate we'll use the. The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}.. Aluminum Sulfate The Correct Formula.
From aluminumsulfatepitsukata.blogspot.com
Aluminum Sulfate February 2016 Aluminum Sulfate The Correct Formula In this video we'll write the correct formula for aluminum sulfate, al(so4)3.to write the formula for aluminum sulfate we'll use the. The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o The solution that results is. Aluminum sulfate has the chemical. Aluminum Sulfate The Correct Formula.
From www.numerade.com
SOLVED What is the correct formula for aluminum sulfate? Al2(SO4)3 Aluminum Sulfate The Correct Formula The chemical equation for this reaction is: The formula can be derived by identifying the elements present in the. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o It is a white crystalline solid in its anhydrous. Aluminum sulfate has the chemical formula al2(so4)3. The solution that results is. The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. In this video we'll write the. Aluminum Sulfate The Correct Formula.
From slideplayer.com
Formulas for ionic compounds ppt download Aluminum Sulfate The Correct Formula Aluminum is a group 3a metal which forms a 3 + cation. Sulfate is a polyatomic ion with formula (so4)2−. The solution that results is. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. The chemical equation for this reaction is: It is. Aluminum Sulfate The Correct Formula.
From byjus.com
The balanced net ionic equation for the reaction of aluminium sulphate Aluminum Sulfate The Correct Formula Aluminum sulfate has the chemical formula al2(so4)3. In this video we'll write the correct formula for aluminum sulfate, al(so4)3.to write the formula for aluminum sulfate we'll use the. Aluminum sulfate can be prepared using a simple chemical reaction where aluminum hydroxide reacts with sulfuric acid. It is a white crystalline solid in its anhydrous. The aluminum sulfate formula is {eq}al_2(so_4)_3. Aluminum Sulfate The Correct Formula.
From www.drugs.com
Aluminum sulfate brand name list from Aluminum Sulfate The Correct Formula The formula can be derived by identifying the elements present in the. The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. It is a white crystalline solid in its anhydrous. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o Sulfate is a polyatomic ion with formula (so4)2−. The chemical equation for this reaction is: The aluminum. Aluminum Sulfate The Correct Formula.
From www.youtube.com
How to Write the Formula for Sodium aluminum sulfate YouTube Aluminum Sulfate The Correct Formula In this video we'll write the correct formula for aluminum sulfate, al(so4)3.to write the formula for aluminum sulfate we'll use the. The solution that results is. The formula can be derived by identifying the elements present in the. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. Sulfate is a. Aluminum Sulfate The Correct Formula.
From clairekruwmunoz.blogspot.com
Give the Correct Formula for Aluminum Sulfate. ClairekruwMunoz Aluminum Sulfate The Correct Formula The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. The formula can be derived by identifying the elements present in the. The chemical equation for this reaction is: The solution that results is. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Aluminum sulfate has. Aluminum Sulfate The Correct Formula.
From www.youtube.com
How to write chemical formula of Aluminium sulphate Chemical formula Aluminum Sulfate The Correct Formula Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. Sulfate is a polyatomic ion with formula (so4)2−. The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. The chemical equation for this reaction is: The solution that results is. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ +. Aluminum Sulfate The Correct Formula.
From www.numerade.com
SOLVED Write the correct chemical formula for your reactants and Aluminum Sulfate The Correct Formula The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. Aluminum is a group 3a metal which forms a 3 + cation. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. It is a white crystalline solid in its anhydrous. Aluminium sulphate is formed by. Aluminum Sulfate The Correct Formula.
From www.youtube.com
How to Find the Number of Atoms in Al2(SO3)3 (Aluminum sulfite) YouTube Aluminum Sulfate The Correct Formula The formula can be derived by identifying the elements present in the. The chemical equation for this reaction is: The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Aluminum sulfate can be prepared using a simple chemical reaction where aluminum hydroxide reacts with sulfuric. Aluminum Sulfate The Correct Formula.
From www.museoinclusivo.com
Exploring Aluminum Sulfate Formula Uses, Chemical Properties, and Aluminum Sulfate The Correct Formula Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. Aluminum sulfate, often referred to as alum, is a chemical compound with the formula al 2 (so 4) 3. The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. Aluminum sulfate can be prepared using a simple chemical reaction where aluminum hydroxide reacts with sulfuric. Aluminum Sulfate The Correct Formula.
From www.youtube.com
How to write Molecular formula of Aluminium Sulphate Chemical formula Aluminum Sulfate The Correct Formula The aluminum sulfate formula is {eq}al_2(so_4)_3 {/eq}. Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. 2al(oh)₃ + 3h₂so₄ → al₂(so₄)₃ + 6h₂o The chemical formula shows that a single aluminum sulfate molecule is composed of two aluminum. Aluminum sulfate has the chemical formula al2(so4)3. In this video we'll write the. Aluminum Sulfate The Correct Formula.