Titration Graph Endpoint . if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in color, which is called the endpoint of the indicator. finding the endpoint of a titration. a titration curve is a graphical representation of the ph of a solution during a titration. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. The end point of a titration is the point at. You will also learn about equivalence points and endpoints, and titration calculations. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the.
from general.chemistrysteps.com
For a ph titration (between an acid and a base), there are two common ways to find the endpoint: titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. finding the endpoint of a titration. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. You will also learn about equivalence points and endpoints, and titration calculations. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. The end point of a titration is the point at. a titration curve is a graphical representation of the ph of a solution during a titration. if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in color, which is called the endpoint of the indicator.
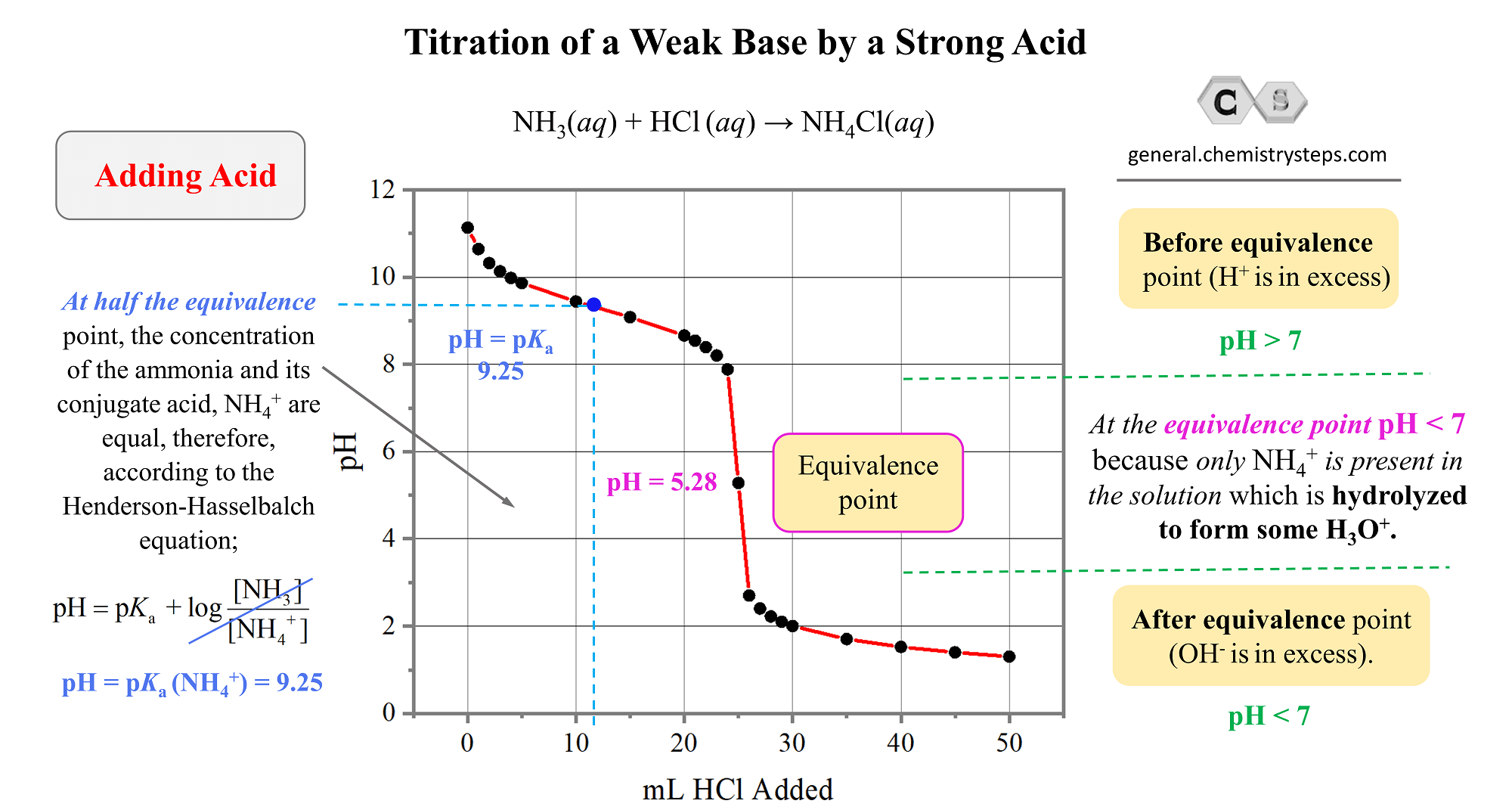
Titration of a Weak Base by a Strong Acid Chemistry Steps
Titration Graph Endpoint titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. a titration curve is a graphical representation of the ph of a solution during a titration. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. You will also learn about equivalence points and endpoints, and titration calculations. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in color, which is called the endpoint of the indicator. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. finding the endpoint of a titration. The end point of a titration is the point at.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Graph Endpoint For a ph titration (between an acid and a base), there are two common ways to find the endpoint: in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration curve is a graphical representation of the ph of a solution during a titration. You will also learn about. Titration Graph Endpoint.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Graph Endpoint if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in color, which is called the endpoint of the indicator. finding the endpoint of a titration. You will also learn about equivalence points and endpoints, and titration calculations. a titration curve is a graphical representation of the ph. Titration Graph Endpoint.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Graph Endpoint The end point of a titration is the point at. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in color, which is called the endpoint of the indicator.. Titration Graph Endpoint.
From animalia-life.club
Endpoint Titration Titration Graph Endpoint You will also learn about equivalence points and endpoints, and titration calculations. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. The end point of a titration is the point at. finding the endpoint of a titration. For a ph titration (between an acid and a base), there are. Titration Graph Endpoint.
From narodnatribuna.info
Ppt How To Interpret Titration Curves Powerpoint Titration Graph Endpoint For a ph titration (between an acid and a base), there are two common ways to find the endpoint: You will also learn about equivalence points and endpoints, and titration calculations. a titration curve is a graphical representation of the ph of a solution during a titration. titration curves show how the ph of an acidic or basic. Titration Graph Endpoint.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Graph Endpoint You will also learn about equivalence points and endpoints, and titration calculations. The end point of a titration is the point at. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform. Titration Graph Endpoint.
From mungfali.com
Endpoint Titration Curve Titration Graph Endpoint titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. finding the endpoint of a titration. a titration curve is a graphical representation of the ph of a solution during a titration. For a ph titration (between an acid and a base), there are two common ways to. Titration Graph Endpoint.
From www.animalia-life.club
Endpoint Titration Titration Graph Endpoint in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added. Titration Graph Endpoint.
From animalia-life.club
Endpoint Titration Titration Graph Endpoint a titration curve is a graphical representation of the ph of a solution during a titration. if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in color, which is called the endpoint of the indicator. finding the endpoint of a titration. The end point of a titration. Titration Graph Endpoint.
From www.youtube.com
Acid Base Titrations, pH Curves and Endpoints YouTube Titration Graph Endpoint a titration curve is a graphical representation of the ph of a solution during a titration. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. in this tutorial,. Titration Graph Endpoint.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Graph Endpoint finding the endpoint of a titration. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. a titration curve is a graphical representation of the ph of a solution during a titration. if a few drops of indicator solution have been added, this sharp increase in ph. Titration Graph Endpoint.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Graph Endpoint The end point of a titration is the point at. a titration curve is a graphical representation of the ph of a solution during a titration. if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in color, which is called the endpoint of the indicator. in this. Titration Graph Endpoint.
From www.animalia-life.club
Endpoint Titration Titration Graph Endpoint a titration curve is a graphical representation of the ph of a solution during a titration. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one.. Titration Graph Endpoint.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Graph Endpoint The end point of a titration is the point at. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration curve is a graphical representation of the ph. Titration Graph Endpoint.
From www.animalia-life.club
Endpoint Titration Titration Graph Endpoint if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in color, which is called the endpoint of the indicator. The end point of a titration is the point at. You will also learn about equivalence points and endpoints, and titration calculations. finding the endpoint of a titration. . Titration Graph Endpoint.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Graph Endpoint in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration curve is a graphical representation of the ph of a solution during a titration. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it.. Titration Graph Endpoint.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Graph Endpoint finding the endpoint of a titration. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in color, which is called the endpoint of the indicator. a titration curve. Titration Graph Endpoint.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Graph Endpoint a titration curve is a graphical representation of the ph of a solution during a titration. finding the endpoint of a titration. The end point of a titration is the point at. You will also learn about equivalence points and endpoints, and titration calculations. titration curves show how the ph of an acidic or basic solution changes. Titration Graph Endpoint.
From www.animalia-life.club
Endpoint Titration Titration Graph Endpoint titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: finding the endpoint of a titration. in this tutorial, you will learn about titration curves,. Titration Graph Endpoint.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Graph Endpoint titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. For a ph titration (between an acid and a base), there are two common ways to. Titration Graph Endpoint.
From www.youtube.com
Conductometric titration of weak acid and strong base (weak acid vs Titration Graph Endpoint titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in. Titration Graph Endpoint.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Graph Endpoint a titration curve is a graphical representation of the ph of a solution during a titration. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. The end point of a titration is the point at. titration curves show how the ph of an acidic or basic solution. Titration Graph Endpoint.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titration Graph Endpoint titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. You will also learn about equivalence points and endpoints, and titration calculations. a titration curve is a graphical representation of the ph of a solution during a titration. if a few drops of indicator solution have been added,. Titration Graph Endpoint.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Graph Endpoint if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in color, which is called the endpoint of the indicator. finding the endpoint of a titration. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. You will also. Titration Graph Endpoint.
From www.vrogue.co
Endpoint Titration Curve vrogue.co Titration Graph Endpoint in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You will also learn about equivalence points and endpoints, and titration calculations. The end point of a titration is the point at. if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt. Titration Graph Endpoint.
From courses.lumenlearning.com
AcidBase Titrations General Chemistry Titration Graph Endpoint finding the endpoint of a titration. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. The end point of a titration is the point at. a titration curve. Titration Graph Endpoint.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Graph Endpoint titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in color, which is called the endpoint of the indicator. titration curves show how the ph of an acidic. Titration Graph Endpoint.
From www.animalia-life.club
Endpoint Titration Titration Graph Endpoint finding the endpoint of a titration. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: a titration curve is a graphical representation of the ph of a solution. Titration Graph Endpoint.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Graph Endpoint The end point of a titration is the point at. a titration curve is a graphical representation of the ph of a solution during a titration. if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in color, which is called the endpoint of the indicator. For a ph. Titration Graph Endpoint.
From hxeppfxlk.blob.core.windows.net
Titration Equivalence Point Endpoint at Nicole Dubois blog Titration Graph Endpoint titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. You will also learn about equivalence points and endpoints, and titration calculations. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: in this tutorial, you will learn about titration. Titration Graph Endpoint.
From www.writework.com
Titration of amino acids WriteWork Titration Graph Endpoint For a ph titration (between an acid and a base), there are two common ways to find the endpoint: in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in color,. Titration Graph Endpoint.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Graph Endpoint The end point of a titration is the point at. You will also learn about equivalence points and endpoints, and titration calculations. finding the endpoint of a titration. a titration curve is a graphical representation of the ph of a solution during a titration. in this tutorial, you will learn about titration curves, titration analysis and the. Titration Graph Endpoint.
From www.youtube.com
How to Find the Equivalence Point on a Titration Graph In Excel YouTube Titration Graph Endpoint finding the endpoint of a titration. a titration curve is a graphical representation of the ph of a solution during a titration. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: titration curves show how the ph of an acidic or basic solution changes as a basic. Titration Graph Endpoint.
From hxeetojpk.blob.core.windows.net
Sharp End Point Titration at Tyrone McKenna blog Titration Graph Endpoint For a ph titration (between an acid and a base), there are two common ways to find the endpoint: a titration curve is a graphical representation of the ph of a solution during a titration. You will also learn about equivalence points and endpoints, and titration calculations. in this tutorial, you will learn about titration curves, titration analysis. Titration Graph Endpoint.
From www.vrogue.co
Solvedthe Graph Shows The Titration Curv vrogue.co Titration Graph Endpoint a titration curve is a graphical representation of the ph of a solution during a titration. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. if a few drops of indicator solution have been added, this sharp increase in ph causes an abrupt change in color, which is. Titration Graph Endpoint.