Dilution Process Is Accompanied With . dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. understand how stock solutions are used in the laboratory. these relations tell us that the dilution of a substance from an initial concentration \(c_1\) to a more dilute. the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete. It describes the number of moles of a solute that are. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. dilution is a process used to lower the concentration of the original solution by adding more solvent. molarity is a unit of concentration that is expressed as moles per liter (m).
from materialschoolpinder.z21.web.core.windows.net
It describes the number of moles of a solute that are. dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. understand how stock solutions are used in the laboratory. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete. dilution is a process used to lower the concentration of the original solution by adding more solvent. molarity is a unit of concentration that is expressed as moles per liter (m). these relations tell us that the dilution of a substance from an initial concentration \(c_1\) to a more dilute. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent.
How To Do Dilutions In Chemistry
Dilution Process Is Accompanied With dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. these relations tell us that the dilution of a substance from an initial concentration \(c_1\) to a more dilute. the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. molarity is a unit of concentration that is expressed as moles per liter (m). understand how stock solutions are used in the laboratory. It describes the number of moles of a solute that are. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete.
From exofldwwh.blob.core.windows.net
Dilution Method In Medicine at Joni Blomberg blog Dilution Process Is Accompanied With dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. . Dilution Process Is Accompanied With.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Process Is Accompanied With dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. dilution is a process used to lower the concentration of the original solution by adding more solvent. molarity is a unit of concentration that is expressed as moles per liter (m). the process increases the total volume of the. Dilution Process Is Accompanied With.
From ceksdxdt.blob.core.windows.net
Serial Dilution Method Principle at Joe Cunningham blog Dilution Process Is Accompanied With understand how stock solutions are used in the laboratory. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. these relations tell us that the dilution of a substance from an initial concentration \(c_1\) to a more dilute. Muriatic acid, another name for hcl (aq), is widely used for cleaning. Dilution Process Is Accompanied With.
From bio.libretexts.org
15 Determination of Bacterial Numbers Biology LibreTexts Dilution Process Is Accompanied With the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. molarity is a unit of concentration that is expressed as moles per liter (m). understand how stock solutions are used in the laboratory. It describes the number of moles of a solute that are. these relations tell us that. Dilution Process Is Accompanied With.
From www.integra-biosciences.com
Guide Learn how to perform serial dilutions with INTEGRA INTEGRA Dilution Process Is Accompanied With understand how stock solutions are used in the laboratory. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete. dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. the process increases the total volume of the solution while proportionally decreasing the. Dilution Process Is Accompanied With.
From exoaaybok.blob.core.windows.net
Dilution Method Microbiology at Harry Hausman blog Dilution Process Is Accompanied With understand how stock solutions are used in the laboratory. molarity is a unit of concentration that is expressed as moles per liter (m). these relations tell us that the dilution of a substance from an initial concentration \(c_1\) to a more dilute. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete. It describes. Dilution Process Is Accompanied With.
From cejakhtq.blob.core.windows.net
How To Do A Dilution Experiment at Lillian Benda blog Dilution Process Is Accompanied With dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. understand how stock solutions are used in the laboratory. dilution is a process used to lower the concentration of the original solution by adding more solvent. It describes the number of moles of a solute that are. the process. Dilution Process Is Accompanied With.
From cewxohfx.blob.core.windows.net
Dilution And Solution Preparation at Rita Favela blog Dilution Process Is Accompanied With dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. dilution is a process used to lower the concentration of the original solution by adding more solvent. . Dilution Process Is Accompanied With.
From hereditybio.in
Serial Dilution Technique for Bacterial Quantification Procedure and Dilution Process Is Accompanied With dilution is a process used to lower the concentration of the original solution by adding more solvent. understand how stock solutions are used in the laboratory. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete. these relations tell us that the dilution of a substance from an initial concentration \(c_1\) to a more. Dilution Process Is Accompanied With.
From cevxwmet.blob.core.windows.net
Dilution Series Calculation at Darren Burgess blog Dilution Process Is Accompanied With It describes the number of moles of a solute that are. dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. dilution is the process whereby the concentration. Dilution Process Is Accompanied With.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Process Is Accompanied With these relations tell us that the dilution of a substance from an initial concentration \(c_1\) to a more dilute. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. dilution is a process used to lower the concentration of the original solution by adding more solvent. molarity is a. Dilution Process Is Accompanied With.
From cexkiyfv.blob.core.windows.net
How To Do Dilutions In Chemistry at Gussie Jasmin blog Dilution Process Is Accompanied With the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. . Dilution Process Is Accompanied With.
From mmerevise.co.uk
Concentrations and Dilutions MME Dilution Process Is Accompanied With understand how stock solutions are used in the laboratory. dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. molarity is a unit of concentration that is expressed as moles per liter (m). dilution is a process used to lower the concentration of the. Dilution Process Is Accompanied With.
From studypergunnahs.z13.web.core.windows.net
What Is Dilution Effect Dilution Process Is Accompanied With dilution is a process used to lower the concentration of the original solution by adding more solvent. It describes the number of moles of a solute that are. understand how stock solutions are used in the laboratory. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete. these relations tell us that the dilution. Dilution Process Is Accompanied With.
From www.researchgate.net
Set of images showing the dilution process (from left to right) of Dilution Process Is Accompanied With dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. molarity is a unit of concentration that is expressed as moles per liter (m). these relations tell us that the dilution of a substance from an initial concentration \(c_1\) to a more dilute. It describes. Dilution Process Is Accompanied With.
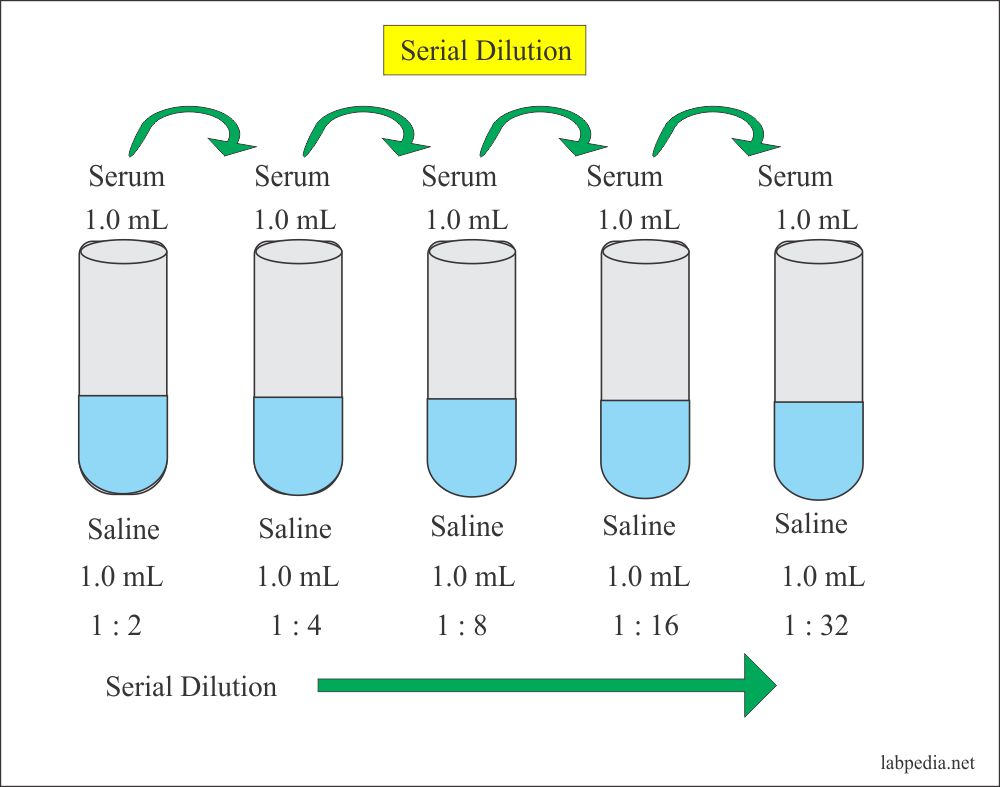
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Process Is Accompanied With dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. dilution is a process used to lower the concentration of the original solution by adding more solvent. . Dilution Process Is Accompanied With.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Process Is Accompanied With dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. It describes the number of moles of a solute that are. these relations tell us that the dilution. Dilution Process Is Accompanied With.
From cevxwmet.blob.core.windows.net
Dilution Series Calculation at Darren Burgess blog Dilution Process Is Accompanied With dilution is a process used to lower the concentration of the original solution by adding more solvent. the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. molarity is a unit of concentration that is expressed as moles per liter (m). It describes the number of moles of a solute. Dilution Process Is Accompanied With.
From mungfali.com
Serial Dilution Steps Dilution Process Is Accompanied With dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. dilution is a process used to lower the concentration of the original solution by adding more solvent. molarity is a unit of concentration that is expressed as moles per liter (m). Muriatic acid, another name. Dilution Process Is Accompanied With.
From exoaaybok.blob.core.windows.net
Dilution Method Microbiology at Harry Hausman blog Dilution Process Is Accompanied With dilution is a process used to lower the concentration of the original solution by adding more solvent. understand how stock solutions are used in the laboratory. the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. dilution is the process of reducing the concentration of a solute in a. Dilution Process Is Accompanied With.
From dxobyjbye.blob.core.windows.net
How To Make Dilutions In Lab at Drew Ballard blog Dilution Process Is Accompanied With understand how stock solutions are used in the laboratory. dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. molarity is a unit of concentration that is expressed as moles per liter (m). dilution is the process whereby the concentration of a solution is. Dilution Process Is Accompanied With.
From prabhakarpk.blogspot.com
Serial Dilution Definition, Formula, Calculator, Procedure, Uses Dilution Process Is Accompanied With molarity is a unit of concentration that is expressed as moles per liter (m). understand how stock solutions are used in the laboratory. dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the process of reducing the concentration of a solute in a solution, usually by. Dilution Process Is Accompanied With.
From www.researchgate.net
Dilution method for the isolation of soil fungi. Download Scientific Dilution Process Is Accompanied With It describes the number of moles of a solute that are. the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the process whereby the concentration of a solution is lessened. Dilution Process Is Accompanied With.
From www.youtube.com
Serial Dilution Technique For Microbiological & Chemical Analysis Dilution Process Is Accompanied With the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. molarity is a unit of concentration that is expressed as moles per liter (m). these relations tell. Dilution Process Is Accompanied With.
From www.researchgate.net
Procedures of serial dilution preparation Download Scientific Diagram Dilution Process Is Accompanied With It describes the number of moles of a solute that are. understand how stock solutions are used in the laboratory. these relations tell us that the dilution of a substance from an initial concentration \(c_1\) to a more dilute. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete. dilution is the process of. Dilution Process Is Accompanied With.
From www.slideserve.com
PPT Lesson 18 PowerPoint Presentation, free download ID3739399 Dilution Process Is Accompanied With the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. It describes the number of moles of a solute that are. dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. dilution is the process whereby the concentration. Dilution Process Is Accompanied With.
From www.youtube.com
Serial Dilution Methods & Calaculations YouTube Dilution Process Is Accompanied With these relations tell us that the dilution of a substance from an initial concentration \(c_1\) to a more dilute. It describes the number of moles of a solute that are. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Muriatic acid, another name for hcl (aq), is widely used for. Dilution Process Is Accompanied With.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Process Is Accompanied With Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete. the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. It describes the number of moles of a solute that are. understand how stock solutions are used in the laboratory. dilution is a process used to lower. Dilution Process Is Accompanied With.
From materialschoolpinder.z21.web.core.windows.net
How To Do Dilutions In Chemistry Dilution Process Is Accompanied With It describes the number of moles of a solute that are. understand how stock solutions are used in the laboratory. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete. these relations tell us that the dilution of a substance from an initial concentration \(c_1\) to a more dilute. dilution is a process used. Dilution Process Is Accompanied With.
From www.studocu.com
Dilutions Lab Two . Dilutions For many years, scientists have used Dilution Process Is Accompanied With Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete. the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. It describes the number of moles of a solute that are.. Dilution Process Is Accompanied With.
From ceksdxdt.blob.core.windows.net
Serial Dilution Method Principle at Joe Cunningham blog Dilution Process Is Accompanied With these relations tell us that the dilution of a substance from an initial concentration \(c_1\) to a more dilute. molarity is a unit of concentration that is expressed as moles per liter (m). dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the process of reducing. Dilution Process Is Accompanied With.
From cexkiyfv.blob.core.windows.net
How To Do Dilutions In Chemistry at Gussie Jasmin blog Dilution Process Is Accompanied With understand how stock solutions are used in the laboratory. dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. molarity is a unit of concentration that is expressed as moles per liter (m). these relations tell us that the dilution of a substance from. Dilution Process Is Accompanied With.
From www.researchgate.net
Agar dilution method with NPs. Download Scientific Diagram Dilution Process Is Accompanied With molarity is a unit of concentration that is expressed as moles per liter (m). It describes the number of moles of a solute that are. Muriatic acid, another name for hcl (aq), is widely used for cleaning concrete. the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. dilution is. Dilution Process Is Accompanied With.
From ceqnefyw.blob.core.windows.net
Dilution Method Of Ast at Clement Meador blog Dilution Process Is Accompanied With It describes the number of moles of a solute that are. understand how stock solutions are used in the laboratory. molarity is a unit of concentration that is expressed as moles per liter (m). dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. . Dilution Process Is Accompanied With.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Process Is Accompanied With the process increases the total volume of the solution while proportionally decreasing the concentration of the solute. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. dilution is the process of reducing the concentration of a solute in a solution, usually by mixing with more of the solvent. . Dilution Process Is Accompanied With.