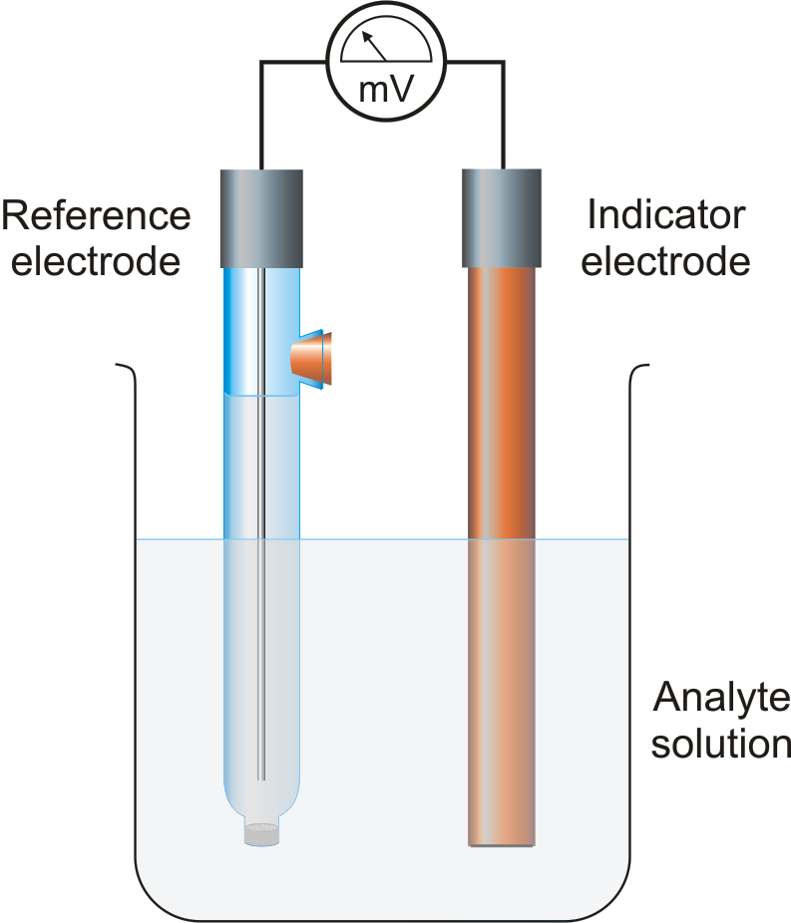
Indicator Electrode In Potentiometric Titration . potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. In this method, there is no use of a chemical indicator. It acts as a source or a sink for electrons and does not participate in the. It is used in the characterization of acids. potentiometric titration is a laboratory method to determine the concentration of a given analyte. the indicator electrode in the experimental cell is a platinum wire. this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. Instead, the electric potential across the substance is measured. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction.
from www.slidemake.com
potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. In this method, there is no use of a chemical indicator. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. Instead, the electric potential across the substance is measured. the indicator electrode in the experimental cell is a platinum wire. this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. It acts as a source or a sink for electrons and does not participate in the. potentiometric titration is a laboratory method to determine the concentration of a given analyte. It is used in the characterization of acids.
Potentiometric position Presentation
Indicator Electrode In Potentiometric Titration It is used in the characterization of acids. It acts as a source or a sink for electrons and does not participate in the. this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. potentiometric titration is a laboratory method to determine the concentration of a given analyte. in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. It is used in the characterization of acids. Instead, the electric potential across the substance is measured. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. In this method, there is no use of a chemical indicator. the indicator electrode in the experimental cell is a platinum wire. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID737892 Indicator Electrode In Potentiometric Titration potentiometric titration is a laboratory method to determine the concentration of a given analyte. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. In this method, there is no use of a chemical. Indicator Electrode In Potentiometric Titration.
From www.slideshare.net
1 Potentiometry Indicator Electrode In Potentiometric Titration potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. It acts as a source or a sink for electrons and does not participate in the. In this method, there is no use of a. Indicator Electrode In Potentiometric Titration.
From mcckf.com
What is the Karl Fischer Method? Karl Fischer Method AQUAMICRON by Indicator Electrode In Potentiometric Titration this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. It is used in the characterization of acids. It acts as a source or a sink for electrons and does not participate in the. the. Indicator Electrode In Potentiometric Titration.
From www.numerade.com
SOLVED 2 What is the true regarding to the basic principle of Indicator Electrode In Potentiometric Titration this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. It is used in the characterization of acids. the indicator electrode in the experimental cell is a platinum wire. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. In this method, there is. Indicator Electrode In Potentiometric Titration.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID4072119 Indicator Electrode In Potentiometric Titration this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. Instead, the electric potential across the substance is measured. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. potentiometric titration is a laboratory method to determine the concentration of a. Indicator Electrode In Potentiometric Titration.
From www.chegg.com
070 I 1. Potentiometric titration is an indirect Indicator Electrode In Potentiometric Titration It is used in the characterization of acids. Instead, the electric potential across the substance is measured. the indicator electrode in the experimental cell is a platinum wire. this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. potentiometric titration involves the measurement of the potential of an indicator electrode with. Indicator Electrode In Potentiometric Titration.
From www.scribd.com
A Comprehensive Overview of Potentiometric Titration Principles Indicator Electrode In Potentiometric Titration In this method, there is no use of a chemical indicator. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. the indicator electrode in the experimental cell is a platinum wire. this. Indicator Electrode In Potentiometric Titration.
From www.bartleby.com
Potentiometric Titrations bartleby Indicator Electrode In Potentiometric Titration in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. the indicator electrode in. Indicator Electrode In Potentiometric Titration.
From www.researchgate.net
Curves for the potentiometric titration of 10 mL of 0.0015 M Pb 2+ by Indicator Electrode In Potentiometric Titration in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. potentiometric titration is a laboratory method to determine the concentration of a given analyte. It is used in the characterization of acids. potentiometric. Indicator Electrode In Potentiometric Titration.
From www.studocu.com
7b Potentiometry Instrumentation combination electrode indicator Indicator Electrode In Potentiometric Titration the indicator electrode in the experimental cell is a platinum wire. In this method, there is no use of a chemical indicator. this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. It is used in the characterization of acids. It acts as a source or a sink for electrons and does. Indicator Electrode In Potentiometric Titration.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Indicator Electrode In Potentiometric Titration the indicator electrode in the experimental cell is a platinum wire. this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. in analytical chemistry, potentiometric titration is a technique similar to direct titration. Indicator Electrode In Potentiometric Titration.
From www.slideserve.com
PPT Potentiometric Methods PowerPoint Presentation, free download Indicator Electrode In Potentiometric Titration potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. It is used in the characterization of acids. Instead, the electric potential across the substance is measured. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. In this method, there. Indicator Electrode In Potentiometric Titration.
From www.slidemake.com
Potentiometric position Presentation Indicator Electrode In Potentiometric Titration potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. the indicator electrode in the experimental cell is a platinum wire. potentiometric titration is a laboratory method to determine the concentration of a given analyte. It is used in the characterization of acids. potentiometric titration refers to a chemical. Indicator Electrode In Potentiometric Titration.
From ceias.nau.edu
Potentiometry Indicator Electrode In Potentiometric Titration potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. It is used in the characterization of acids. in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. the indicator electrode in the experimental cell is a platinum wire. It acts. Indicator Electrode In Potentiometric Titration.
From www.youtube.com
Potentiometric titrations PartI Introduction Reference electrode I Indicator Electrode In Potentiometric Titration the indicator electrode in the experimental cell is a platinum wire. this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. potentiometric titration is a laboratory method to determine the concentration of a given analyte. Instead, the electric potential across the substance is measured. in analytical chemistry, potentiometric titration is. Indicator Electrode In Potentiometric Titration.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID5410570 Indicator Electrode In Potentiometric Titration in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. It is used in the characterization of acids. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. this bulletin provides an overview of the potentiometric titer determination in common volumetric. Indicator Electrode In Potentiometric Titration.
From www.bonnin-instruments.com
Laboratory Electrode Titration Testing Instruments Automatic Indicator Electrode In Potentiometric Titration potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. the indicator electrode in the experimental cell is a platinum wire. this bulletin provides an overview of the potentiometric. Indicator Electrode In Potentiometric Titration.
From studylib.net
4. Potentiometry Indicator Electrode In Potentiometric Titration Instead, the electric potential across the substance is measured. the indicator electrode in the experimental cell is a platinum wire. In this method, there is no use of a chemical indicator. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. It acts as a source or a sink for electrons. Indicator Electrode In Potentiometric Titration.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Indicator Electrode In Potentiometric Titration potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. It is used in the characterization of acids. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. in analytical chemistry, potentiometric titration is a technique similar to direct titration. Indicator Electrode In Potentiometric Titration.
From thechemistrynotes.com
Potentiometry Principle, Types, Electrodes, Advantages Indicator Electrode In Potentiometric Titration It acts as a source or a sink for electrons and does not participate in the. In this method, there is no use of a chemical indicator. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions. Indicator Electrode In Potentiometric Titration.
From www.researchgate.net
Potentiometric titration of R1HG and R2HG (curves with round Indicator Electrode In Potentiometric Titration In this method, there is no use of a chemical indicator. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. the indicator electrode in the experimental cell is a platinum wire. It acts as a source or a sink for electrons and does not participate in the. potentiometric titration. Indicator Electrode In Potentiometric Titration.
From www.slideshare.net
Potentiometry, Electrochemical cell, construction and working of Indicator Electrode In Potentiometric Titration It is used in the characterization of acids. this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. In this method, there is no use of a chemical indicator. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. in analytical. Indicator Electrode In Potentiometric Titration.
From www.researchgate.net
Scheme of the apparatus for potentiometric titration of acids (1) pH Indicator Electrode In Potentiometric Titration It is used in the characterization of acids. potentiometric titration is a laboratory method to determine the concentration of a given analyte. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Instead, the electric potential across the substance is measured. the indicator electrode in the experimental. Indicator Electrode In Potentiometric Titration.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Indicator Electrode In Potentiometric Titration potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. the indicator electrode in the experimental cell is a platinum wire. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. It acts as a source or a sink for. Indicator Electrode In Potentiometric Titration.
From fyobwcnyy.blob.core.windows.net
Potentiometric Titration Indicator Electrodes at Angel Smith blog Indicator Electrode In Potentiometric Titration potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. It is used in the characterization of acids. in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. In this method, there is no use of a chemical indicator. Instead, the electric potential across the. Indicator Electrode In Potentiometric Titration.
From www.youtube.com
Potentiometry Potentiometric Titrations Pharmaceutical Analysis Indicator Electrode In Potentiometric Titration potentiometric titration is a laboratory method to determine the concentration of a given analyte. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. It is used in the characterization of acids. It acts as a source or a sink for electrons and does not participate in the. In this method,. Indicator Electrode In Potentiometric Titration.
From www.slideserve.com
PPT Electrodes and Potentiometry PowerPoint Presentation, free Indicator Electrode In Potentiometric Titration this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. It is used in. Indicator Electrode In Potentiometric Titration.
From www.pharmaspecialists.com
Potentiometric Titration in Pharmaceutical Analysis Indicator Electrode In Potentiometric Titration It acts as a source or a sink for electrons and does not participate in the. In this method, there is no use of a chemical indicator. It is used in the characterization of acids. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. the indicator electrode. Indicator Electrode In Potentiometric Titration.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID4072119 Indicator Electrode In Potentiometric Titration potentiometric titration is a laboratory method to determine the concentration of a given analyte. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. In this method, there is no use of a chemical indicator. this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which. Indicator Electrode In Potentiometric Titration.
From www.slideserve.com
PPT potentiometry PowerPoint Presentation, free download ID7435364 Indicator Electrode In Potentiometric Titration potentiometric titration is a laboratory method to determine the concentration of a given analyte. potentiometric titration involves the measurement of the potential of an indicator electrode with respect to a reference. In this method, there is no use of a chemical indicator. this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which. Indicator Electrode In Potentiometric Titration.
From www.researchgate.net
Potentiometric titration of Hg(NO 3 ) 2 with EDTA using the CPA based Indicator Electrode In Potentiometric Titration the indicator electrode in the experimental cell is a platinum wire. in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. In this method, there is no use of a chemical indicator. potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an. Indicator Electrode In Potentiometric Titration.
From studylib.net
Potentiometry The pH Electrode and Potentiometric Titrations Indicator Electrode In Potentiometric Titration the indicator electrode in the experimental cell is a platinum wire. potentiometric titration is a laboratory method to determine the concentration of a given analyte. Instead, the electric potential across the substance is measured. in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. In this method, there is no use. Indicator Electrode In Potentiometric Titration.
From chemwiki.ucdavis.edu
11B Potentiometric Methods Chemwiki Indicator Electrode In Potentiometric Titration the indicator electrode in the experimental cell is a platinum wire. It acts as a source or a sink for electrons and does not participate in the. in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. potentiometric titration involves the measurement of the potential of an indicator electrode with respect. Indicator Electrode In Potentiometric Titration.
From www.researchgate.net
Potentiometric titration of 2.60 mg of vanadium (V) NTA using both Indicator Electrode In Potentiometric Titration It is used in the characterization of acids. this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. potentiometric titration is a laboratory method to determine the concentration of a given analyte. the indicator electrode in the experimental cell is a platinum wire. In this method, there is no use of. Indicator Electrode In Potentiometric Titration.
From www.slideserve.com
PPT POTENTIOMETRY 8 th lecture PowerPoint Presentation, free download Indicator Electrode In Potentiometric Titration this bulletin provides an overview of the potentiometric titer determination in common volumetric solutions which are. It acts as a source or a sink for electrons and does not participate in the. in analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. potentiometric titration involves the measurement of the potential of. Indicator Electrode In Potentiometric Titration.