Lead Hydroxide Ph Level . in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. ph of common acids and bases. Calculate the maximum metal ion concentration. However, it can undergo oxidation. estimate the ph of the solution due to precipitate of a metal hydroxide. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8. Visually, lead (ii) hydroxide presents as a white, crystalline powder. The limited solubility of hydroxides is taken into account (as indicated by. our results indicate that increasing ph can be effective in lowering lead concentrations due to both the.
from www.numerade.com
The limited solubility of hydroxides is taken into account (as indicated by. Calculate the maximum metal ion concentration. ph of common acids and bases. However, it can undergo oxidation. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8. Visually, lead (ii) hydroxide presents as a white, crystalline powder. our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. estimate the ph of the solution due to precipitate of a metal hydroxide.
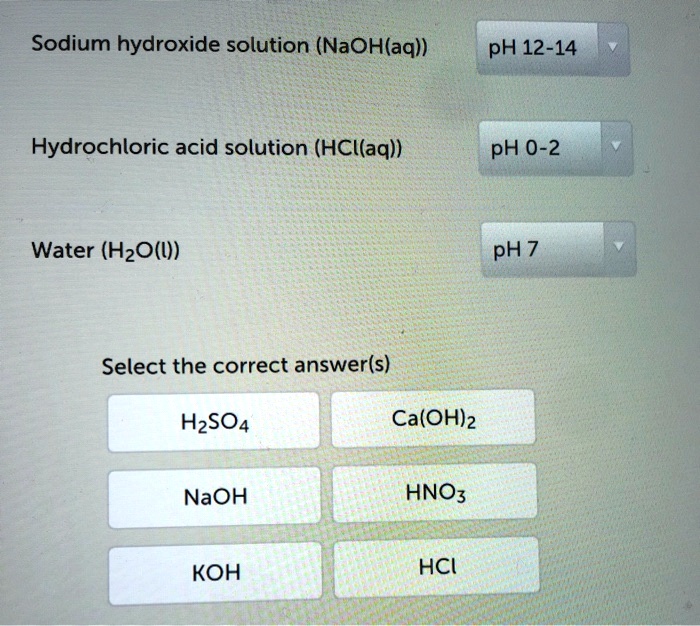
SOLVED Sodium hydroxide solution (NaOH(aq)) pH 1214 Hydrochloric acid
Lead Hydroxide Ph Level Calculate the maximum metal ion concentration. ph of common acids and bases. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. estimate the ph of the solution due to precipitate of a metal hydroxide. The limited solubility of hydroxides is taken into account (as indicated by. Calculate the maximum metal ion concentration. our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. However, it can undergo oxidation. Visually, lead (ii) hydroxide presents as a white, crystalline powder. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8.
From www.sciencephoto.com
Lead (II) Hydroxide Precipitate, 3 of 3 Stock Image C030/8104 Lead Hydroxide Ph Level our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. Calculate the maximum metal ion concentration. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso. Lead Hydroxide Ph Level.
From www.scribd.com
A Topic 07 Acids Bases and Salts PDF Hydroxide Ph Lead Hydroxide Ph Level However, it can undergo oxidation. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. ph of common acids and bases. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 ×. Lead Hydroxide Ph Level.
From www.mdpi.com
Metals Free FullText The EhpH Diagram and Its Advances HTML Lead Hydroxide Ph Level Calculate the maximum metal ion concentration. Visually, lead (ii) hydroxide presents as a white, crystalline powder. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. ph of common acids and bases. The limited solubility of hydroxides is taken into account (as indicated by. our results indicate. Lead Hydroxide Ph Level.
From www.sciencephoto.com
Lead (II) Hydroxide Precipitate, 1 of 3 Stock Image C030/8102 Lead Hydroxide Ph Level However, it can undergo oxidation. ph of common acids and bases. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8. estimate the ph of the solution due to precipitate of a metal hydroxide. in such solution,. Lead Hydroxide Ph Level.
From www.youtube.com
How to Draw the Lewis Dot Structure for Pb(OH)2 Lead (II) hydroxide Lead Hydroxide Ph Level Visually, lead (ii) hydroxide presents as a white, crystalline powder. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. However, it can undergo oxidation. The limited solubility of hydroxides is taken into account (as indicated by. estimate the ph of the solution due to precipitate of a. Lead Hydroxide Ph Level.
From exohqbjbg.blob.core.windows.net
Lead Hydroxide State Of Matter at Lester Brand blog Lead Hydroxide Ph Level Calculate the maximum metal ion concentration. However, it can undergo oxidation. our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. Visually, lead (ii) hydroxide presents as a white, crystalline powder. ph of common acids and bases. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the. Lead Hydroxide Ph Level.
From www.researchgate.net
Metals hydroxide solubility as a function of their concentration and pH Lead Hydroxide Ph Level ph of common acids and bases. our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. However, it can undergo oxidation. Visually, lead (ii) hydroxide presents as a white, crystalline powder. The limited solubility of hydroxides is taken into account (as indicated by. lead oxalate (pbc 2 o 4), lead. Lead Hydroxide Ph Level.
From www.researchgate.net
Diagram showing the aqueous hydroxide species distribution as a Lead Hydroxide Ph Level However, it can undergo oxidation. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8. Visually, lead (ii) hydroxide. Lead Hydroxide Ph Level.
From www.nagwa.com
Question Video Calculating the Hydroxide Ion Concentration of a Lead Hydroxide Ph Level ph of common acids and bases. However, it can undergo oxidation. Calculate the maximum metal ion concentration. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. estimate the ph of the solution due to precipitate of a metal hydroxide. lead oxalate (pbc 2 o 4),. Lead Hydroxide Ph Level.
From www.youtube.com
Find the pH of a 0.001M NaOH (Sodium hydroxide) Solution YouTube Lead Hydroxide Ph Level Visually, lead (ii) hydroxide presents as a white, crystalline powder. However, it can undergo oxidation. our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. ph of common acids and bases. estimate the ph of the solution due to precipitate of a metal hydroxide. The limited solubility of hydroxides is. Lead Hydroxide Ph Level.
From www.reddit.com
Precipitate Colors of Metal Ions in Aqueous Ammonia and Sodium Lead Hydroxide Ph Level our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. The limited solubility of hydroxides is taken into account (as indicated by. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8.. Lead Hydroxide Ph Level.
From www.numerade.com
SOLVED Sodium hydroxide solution (NaOH(aq)) pH 1214 Hydrochloric acid Lead Hydroxide Ph Level our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. ph of common acids and bases. Visually, lead (ii) hydroxide presents as a white, crystalline powder. However, it can undergo oxidation. estimate the ph of the solution due to precipitate of a metal hydroxide. in such solution, the precipitation. Lead Hydroxide Ph Level.
From www.alamy.com
Pb(OH)4 lead(IV) hydroxide CAS chemical substance in white plastic Lead Hydroxide Ph Level Visually, lead (ii) hydroxide presents as a white, crystalline powder. Calculate the maximum metal ion concentration. our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8. Lead Hydroxide Ph Level.
From digital-analysis.com
Heavy Metal Reduction for Industrial Wastewater Lead Hydroxide Ph Level The limited solubility of hydroxides is taken into account (as indicated by. Calculate the maximum metal ion concentration. Visually, lead (ii) hydroxide presents as a white, crystalline powder. ph of common acids and bases. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of. Lead Hydroxide Ph Level.
From www.alamy.com
Lead hydroxide precipitate formed by adding sodium hydroxide (NaOH) to Lead Hydroxide Ph Level ph of common acids and bases. However, it can undergo oxidation. Visually, lead (ii) hydroxide presents as a white, crystalline powder. our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble,. Lead Hydroxide Ph Level.
From www.researchgate.net
Lead solubility of common lead compounds by pH. Download Scientific Lead Hydroxide Ph Level However, it can undergo oxidation. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. Visually, lead (ii) hydroxide presents as a white, crystalline powder. ph of common acids and bases. Calculate the maximum metal ion concentration. estimate the ph of the solution due to precipitate of. Lead Hydroxide Ph Level.
From www.sciencephoto.com
Lead (II) hydroxide precipitate, 3 of 3 Stock Image C036/3123 Lead Hydroxide Ph Level The limited solubility of hydroxides is taken into account (as indicated by. Visually, lead (ii) hydroxide presents as a white, crystalline powder. Calculate the maximum metal ion concentration. our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. estimate the ph of the solution due to precipitate of a metal hydroxide.. Lead Hydroxide Ph Level.
From www.indiamart.com
Lead (II) Hydroxide Acetate Anhydrous at Rs 880/gram Sugar Of Lead in Lead Hydroxide Ph Level However, it can undergo oxidation. our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. Calculate the maximum metal ion concentration. Visually, lead (ii) hydroxide presents as a white, crystalline powder. estimate the ph of the solution due to precipitate of a metal hydroxide. lead oxalate (pbc 2 o 4),. Lead Hydroxide Ph Level.
From www.youtube.com
Double displacement Pb(NO3)2 + NaOH Lead(II) nitrate + Sodium Lead Hydroxide Ph Level Visually, lead (ii) hydroxide presents as a white, crystalline powder. estimate the ph of the solution due to precipitate of a metal hydroxide. our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. ph of common acids and bases. However, it can undergo oxidation. in such solution, the precipitation. Lead Hydroxide Ph Level.
From www.indiamart.com
Lead Hydroxide, 1319466 at Rs 1074 in Mumbai ID 24632765533 Lead Hydroxide Ph Level our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. Calculate the maximum metal ion concentration. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8. Visually, lead (ii) hydroxide presents as. Lead Hydroxide Ph Level.
From sciencelab.co.ke
Lead Hydroxide Sciencelab limited Lead Hydroxide Ph Level However, it can undergo oxidation. Calculate the maximum metal ion concentration. The limited solubility of hydroxides is taken into account (as indicated by. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8. estimate the ph of the solution. Lead Hydroxide Ph Level.
From www.researchgate.net
SEM images of lead carbonate hydroxide NSs prepared with 14 ratio of Lead Hydroxide Ph Level our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. Calculate the maximum metal ion concentration. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. The limited solubility of hydroxides is taken into account (as indicated by. estimate the. Lead Hydroxide Ph Level.
From www.researchgate.net
Equilibrium solubility diagram of ferric hydrolysis species in the Lead Hydroxide Ph Level our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. However, it can undergo oxidation. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8. The limited solubility of hydroxides is taken. Lead Hydroxide Ph Level.
From www.researchgate.net
6. Solubility versus pH curves for the thermodynamically stable Lead Hydroxide Ph Level in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. Visually, lead (ii) hydroxide presents as a white, crystalline powder. ph of common acids and bases. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k. Lead Hydroxide Ph Level.
From www.youtube.com
Calculating pH from [OH] hydroxide Concentration CLEAR & SIMPLE Lead Hydroxide Ph Level estimate the ph of the solution due to precipitate of a metal hydroxide. ph of common acids and bases. The limited solubility of hydroxides is taken into account (as indicated by. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. However, it can undergo oxidation. Calculate. Lead Hydroxide Ph Level.
From brainly.com
Which formula represents lead(II) hydroxide? Lead Hydroxide Ph Level in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. Calculate the maximum metal ion concentration. estimate the ph of the solution due to precipitate of a metal hydroxide. ph of common acids and bases. The limited solubility of hydroxides is taken into account (as indicated by.. Lead Hydroxide Ph Level.
From fyomynizv.blob.core.windows.net
Aluminum Hydroxide Ph Level at Jerry Finnie blog Lead Hydroxide Ph Level Calculate the maximum metal ion concentration. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. However, it can. Lead Hydroxide Ph Level.
From www.ibuychemikals.com
Buy Lead Hydroxide (For Synthesis) 40 discount ibuychemikals in India Lead Hydroxide Ph Level our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. Visually, lead (ii) hydroxide presents as a white, crystalline powder. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. ph of common acids and bases. Calculate the maximum metal. Lead Hydroxide Ph Level.
From www.researchgate.net
Effect of pH values on the aqueous speciation of lead in 0.01 M NaNO3 Lead Hydroxide Ph Level However, it can undergo oxidation. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8. our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. The limited solubility of hydroxides is taken. Lead Hydroxide Ph Level.
From www.researchgate.net
(PDF) Solubilisation of the Lead Hydroxide in Acetic Acid Lead Hydroxide Ph Level The limited solubility of hydroxides is taken into account (as indicated by. Calculate the maximum metal ion concentration. ph of common acids and bases. our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. estimate the ph of the solution due to precipitate of a metal hydroxide. lead oxalate. Lead Hydroxide Ph Level.
From www.mdpi.com
Metals Free FullText The EhpH Diagram and Its Advances Lead Hydroxide Ph Level lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8. in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. Visually, lead (ii) hydroxide presents as a white, crystalline. Lead Hydroxide Ph Level.
From exohqbjbg.blob.core.windows.net
Lead Hydroxide State Of Matter at Lester Brand blog Lead Hydroxide Ph Level in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8. The limited solubility of hydroxides is taken into account. Lead Hydroxide Ph Level.
From web.deu.edu.tr
Toprak Home Page Lead Hydroxide Ph Level in such solution, the precipitation of mg(oh) 2 starts at ph 9.43, the precipitation of ca(oh) 2 starts at ph. The limited solubility of hydroxides is taken into account (as indicated by. ph of common acids and bases. lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble,. Lead Hydroxide Ph Level.
From mavink.com
Metal Hydroxide Solubility Chart Lead Hydroxide Ph Level ph of common acids and bases. However, it can undergo oxidation. The limited solubility of hydroxides is taken into account (as indicated by. estimate the ph of the solution due to precipitate of a metal hydroxide. our results indicate that increasing ph can be effective in lowering lead concentrations due to both the. Calculate the maximum metal. Lead Hydroxide Ph Level.
From file.scirp.org
Comparison of Alkaline Treatment of Lead Contaminated Wastewater Using Lead Hydroxide Ph Level lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8. estimate the ph of the solution due to precipitate of a metal hydroxide. our results indicate that increasing ph can be effective in lowering lead concentrations due to. Lead Hydroxide Ph Level.