Acid Rain Dissolve Marble . Protective coatings, pollution control, and using more resistant materials can help mitigate the effects. The dissolved aluminum begins to accumulate and. Acid rain stains and etches granite and corrodes metals like bronze. This can lead to structural damage and loss of aesthetic detail. Acid precipitation affects stone primarily in two ways: Acid rain damages buildings by corroding metals and dissolving stone, especially limestone and marble. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Acid rain damages structures such as the taj mahal and thomas jefferson memorial. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Thus we can write the reaction of limestone or marble with dilute sulfuric acid as follows: Acid rain can dissolve certain more soluble elements from the soil, like aluminum. Sulfur dioxide, an acid rain precursor, can react directly with limestone in the presence of water to form gypsum, which eventually flakes off or is dissolved by water. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. Acid rain dissolves limestone, marble, cement and sandstone.
from www.chegg.com
Acid rain damages buildings by corroding metals and dissolving stone, especially limestone and marble. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. Acid rain stains and etches granite and corrodes metals like bronze. Acid rain damages structures such as the taj mahal and thomas jefferson memorial. Protective coatings, pollution control, and using more resistant materials can help mitigate the effects. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. This can lead to structural damage and loss of aesthetic detail. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Acid rain dissolves limestone, marble, cement and sandstone. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves.
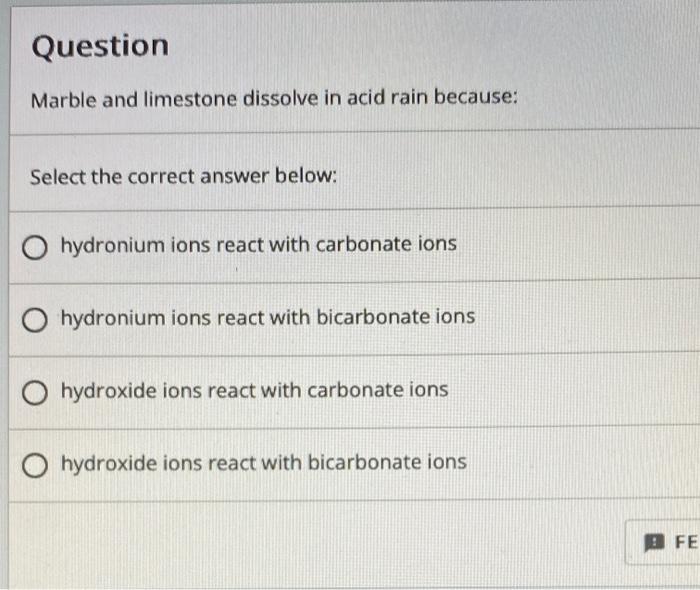
Solved Question Marble and limestone dissolve in acid rain
Acid Rain Dissolve Marble Acid rain damages buildings by corroding metals and dissolving stone, especially limestone and marble. Protective coatings, pollution control, and using more resistant materials can help mitigate the effects. The dissolved aluminum begins to accumulate and. Acid rain damages buildings by corroding metals and dissolving stone, especially limestone and marble. Thus we can write the reaction of limestone or marble with dilute sulfuric acid as follows: Acid precipitation affects stone primarily in two ways: Acid rain stains and etches granite and corrodes metals like bronze. Acid rain dissolves limestone, marble, cement and sandstone. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Acid rain damages structures such as the taj mahal and thomas jefferson memorial. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. This can lead to structural damage and loss of aesthetic detail. Sulfur dioxide, an acid rain precursor, can react directly with limestone in the presence of water to form gypsum, which eventually flakes off or is dissolved by water. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the.
From www.tes.com
Limestone Erosion and Acid Rain Teaching Resources Acid Rain Dissolve Marble Thus we can write the reaction of limestone or marble with dilute sulfuric acid as follows: Acid rain damages structures such as the taj mahal and thomas jefferson memorial. Acid rain stains and etches granite and corrodes metals like bronze. Acid precipitation affects stone primarily in two ways: Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso. Acid Rain Dissolve Marble.
From rogerjcheng.com
ACID RAIN'S EFFECT on MARBLE STRUCTURESANALYTICAL CHEMISTRY Acid Rain Dissolve Marble When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Thus we can write the reaction of limestone or marble with dilute sulfuric acid as follows: Acid rain dissolves limestone, marble, cement and sandstone. Acid precipitation affects stone primarily in two ways: Protective coatings, pollution control, and using more resistant materials can. Acid Rain Dissolve Marble.
From brainly.in
what is meant by acid rain Brainly.in Acid Rain Dissolve Marble When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Acid rain damages buildings by corroding metals and dissolving stone, especially limestone and marble. This can lead to structural damage and loss of aesthetic detail. Sulfur dioxide, an acid rain precursor, can react directly with limestone in the presence of water to. Acid Rain Dissolve Marble.
From www.slideserve.com
PPT Chemical Weathering of Rocks PowerPoint Presentation, free Acid Rain Dissolve Marble Acid rain damages structures such as the taj mahal and thomas jefferson memorial. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Protective coatings, pollution control, and using more resistant materials can help mitigate the effects. When sulfurous, sulfuric, and nitric acids in polluted air react with. Acid Rain Dissolve Marble.
From slideplayer.com
Acid Precipitation. ppt download Acid Rain Dissolve Marble Acid rain stains and etches granite and corrodes metals like bronze. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Acid precipitation affects stone primarily in two ways: The dissolved aluminum begins to accumulate and. Acid rain. Acid Rain Dissolve Marble.
From ar.inspiredpencil.com
Acid Rain Effects On Rocks Acid Rain Dissolve Marble Acid rain damages buildings by corroding metals and dissolving stone, especially limestone and marble. Acid precipitation affects stone primarily in two ways: The dissolved aluminum begins to accumulate and. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4. Acid Rain Dissolve Marble.
From www.researchgate.net
(PDF) Effects of acid rain and sulfur dioxide on marble dissolution Acid Rain Dissolve Marble Acid rain stains and etches granite and corrodes metals like bronze. Acid precipitation affects stone primarily in two ways: Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. When sulfurous, sulfuric, and nitric. Acid Rain Dissolve Marble.
From www.chegg.com
Solved Question Marble and limestone dissolve in acid rain Acid Rain Dissolve Marble When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Protective coatings, pollution control, and using more resistant materials can help mitigate the effects. Acid rain stains and etches granite and corrodes metals like bronze. Sulfur dioxide, an acid rain precursor, can react directly with limestone in the presence of water to. Acid Rain Dissolve Marble.
From www.youtube.com
What's Inside? Dissolving 5 Rocks in Acid YouTube Acid Rain Dissolve Marble Sulfur dioxide, an acid rain precursor, can react directly with limestone in the presence of water to form gypsum, which eventually flakes off or is dissolved by water. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the.. Acid Rain Dissolve Marble.
From www.researchgate.net
Carbonate stone/acid rain phase diagram. Download Scientific Diagram Acid Rain Dissolve Marble Protective coatings, pollution control, and using more resistant materials can help mitigate the effects. Acid rain damages structures such as the taj mahal and thomas jefferson memorial. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because. Acid Rain Dissolve Marble.
From socratic.org
How does acid rain form? Socratic Acid Rain Dissolve Marble The dissolved aluminum begins to accumulate and. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. Acid rain stains and etches granite and corrodes metals like bronze. Acid rain dissolves limestone, marble, cement and sandstone. Acid rain damages buildings by corroding metals and dissolving stone, especially limestone and marble. Protective coatings, pollution control, and using more. Acid Rain Dissolve Marble.
From brainly.ph
1. Refer to figure 1, what effect does acid rain has on Acid Rain Dissolve Marble This can lead to structural damage and loss of aesthetic detail. Acid rain dissolves limestone, marble, cement and sandstone. Thus we can write the reaction of limestone or marble with dilute sulfuric acid as follows: When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. When sulfurous, sulfuric,. Acid Rain Dissolve Marble.
From www.slideserve.com
PPT AcidBase Theories PowerPoint Presentation, free download ID Acid Rain Dissolve Marble Protective coatings, pollution control, and using more resistant materials can help mitigate the effects. Acid rain damages buildings by corroding metals and dissolving stone, especially limestone and marble. Acid precipitation affects stone primarily in two ways: The dissolved aluminum begins to accumulate and. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. Acid rain stains and. Acid Rain Dissolve Marble.
From www.slideserve.com
PPT Weathering PowerPoint Presentation, free download ID2135916 Acid Rain Dissolve Marble Acid rain can dissolve certain more soluble elements from the soil, like aluminum. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Acid rain dissolves limestone, marble, cement and sandstone. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. Acid rain damages. Acid Rain Dissolve Marble.
From www.teachoo.com
Acid Rain Definition, Causes, Effects Teachoo Concepts Acid Rain Dissolve Marble Sulfur dioxide, an acid rain precursor, can react directly with limestone in the presence of water to form gypsum, which eventually flakes off or is dissolved by water. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. Acid rain stains and etches granite and corrodes metals like bronze. This can lead to structural damage and. Acid Rain Dissolve Marble.
From www.dreamstime.com
Marble Stages after a Rain. Stock Image Image of marble, granit Acid Rain Dissolve Marble Acid rain damages buildings by corroding metals and dissolving stone, especially limestone and marble. Protective coatings, pollution control, and using more resistant materials can help mitigate the effects. This can lead to structural damage and loss of aesthetic detail. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. The dissolved aluminum begins to accumulate and.. Acid Rain Dissolve Marble.
From www.slideserve.com
PPT Acid Deposition—Ch 17 PowerPoint Presentation, free download ID Acid Rain Dissolve Marble When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Thus we can write the reaction of limestone or marble with dilute sulfuric acid as follows: Protective coatings, pollution control, and using more resistant materials can help mitigate the effects. Acid rain can dissolve certain more soluble elements. Acid Rain Dissolve Marble.
From www.slideserve.com
PPT Weathering PowerPoint Presentation, free download ID2135916 Acid Rain Dissolve Marble Acid rain dissolves limestone, marble, cement and sandstone. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. This can lead to structural damage and loss of aesthetic detail. Caco3(s) + h2so4(aq) → caso4(s) +. Acid Rain Dissolve Marble.
From www.slideserve.com
PPT Acid Deposition PowerPoint Presentation, free download ID3488645 Acid Rain Dissolve Marble Acid rain stains and etches granite and corrodes metals like bronze. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. Acid rain dissolves limestone, marble, cement and sandstone. Thus we can write the reaction of limestone or marble with dilute sulfuric acid as follows: Sulfur dioxide, an acid rain precursor, can react directly with limestone in. Acid Rain Dissolve Marble.
From slideplayer.com
CHAPTER 25 Acids and Bases. Acids Substances that react with water to Acid Rain Dissolve Marble Sulfur dioxide, an acid rain precursor, can react directly with limestone in the presence of water to form gypsum, which eventually flakes off or is dissolved by water. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the.. Acid Rain Dissolve Marble.
From www.slideserve.com
PPT Weathering and Soil Erosion PowerPoint Presentation, free Acid Rain Dissolve Marble Acid precipitation affects stone primarily in two ways: Acid rain stains and etches granite and corrodes metals like bronze. Acid rain damages structures such as the taj mahal and thomas jefferson memorial. Sulfur dioxide, an acid rain precursor, can react directly with limestone in the presence of water to form gypsum, which eventually flakes off or is dissolved by water.. Acid Rain Dissolve Marble.
From www.perlego.com
[PDF] Evaluation of Heavy Metals from Acid Rain Dissolved StoneCoated Acid Rain Dissolve Marble Acid precipitation affects stone primarily in two ways: Thus we can write the reaction of limestone or marble with dilute sulfuric acid as follows: This can lead to structural damage and loss of aesthetic detail. The dissolved aluminum begins to accumulate and. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. Protective coatings, pollution control,. Acid Rain Dissolve Marble.
From slideplayer.com
Warmup 11/13/12 Weathering is the physical breakdown of rocks. Can Acid Rain Dissolve Marble Acid rain stains and etches granite and corrodes metals like bronze. The dissolved aluminum begins to accumulate and. Acid rain dissolves limestone, marble, cement and sandstone. Acid rain damages buildings by corroding metals and dissolving stone, especially limestone and marble. Protective coatings, pollution control, and using more resistant materials can help mitigate the effects. Thus we can write the reaction. Acid Rain Dissolve Marble.
From fphoto.photoshelter.com
acid rain environment marble atmospheric pollution Fundamental Acid Rain Dissolve Marble Acid rain can dissolve certain more soluble elements from the soil, like aluminum. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. This can lead to structural damage and loss of aesthetic detail. Thus we can write the reaction of limestone or marble with dilute sulfuric acid as follows: Acid rain damages buildings by corroding. Acid Rain Dissolve Marble.
From wwwbrr.cr.usgs.gov
The Erosion of Carbonate Stone by Acid Rain... Acid Rain Dissolve Marble Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. Thus we can write the reaction of limestone or marble with dilute sulfuric acid as follows: Acid rain dissolves limestone, marble, cement and sandstone. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves.. Acid Rain Dissolve Marble.
From www.sliderbase.com
AcidBase Reactions Presentation Chemistry Acid Rain Dissolve Marble Acid rain can dissolve certain more soluble elements from the soil, like aluminum. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. Acid rain damages buildings by corroding metals and dissolving stone, especially limestone and marble. Acid rain damages structures such as the taj mahal and thomas jefferson memorial. Thus we can write the reaction. Acid Rain Dissolve Marble.
From fphoto.photoshelter.com
acid rain environment marble atmospheric pollution Fundamental Acid Rain Dissolve Marble Acid rain stains and etches granite and corrodes metals like bronze. This can lead to structural damage and loss of aesthetic detail. Acid rain damages buildings by corroding metals and dissolving stone, especially limestone and marble. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. Protective coatings, pollution control, and using more resistant materials can help. Acid Rain Dissolve Marble.
From www.slideshare.net
Acid Rain.... Acid Rain Dissolve Marble Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Sulfur dioxide, an acid rain precursor, can react directly with limestone in the presence of water to form gypsum, which eventually flakes off or is dissolved by water.. Acid Rain Dissolve Marble.
From www.slideserve.com
PPT SLOW Processes that Shape the Earth PART 1. WEATHERING PowerPoint Acid Rain Dissolve Marble Acid rain can dissolve certain more soluble elements from the soil, like aluminum. Acid precipitation affects stone primarily in two ways: Sulfur dioxide, an acid rain precursor, can react directly with limestone in the presence of water to form gypsum, which eventually flakes off or is dissolved by water. Acid rain damages structures such as the taj mahal and thomas. Acid Rain Dissolve Marble.
From www.youtube.com
Chemical reaction of marble to acid YouTube Acid Rain Dissolve Marble Acid rain dissolves limestone, marble, cement and sandstone. Acid rain stains and etches granite and corrodes metals like bronze. This can lead to structural damage and loss of aesthetic detail. Acid rain damages buildings by corroding metals and dissolving stone, especially limestone and marble. Acid rain damages structures such as the taj mahal and thomas jefferson memorial. Thus we can. Acid Rain Dissolve Marble.
From www.gutenberg.org
Acid Rain and Our Nation’s Capital, by Elaine McGee a Project Acid Rain Dissolve Marble Acid rain stains and etches granite and corrodes metals like bronze. Sulfur dioxide, an acid rain precursor, can react directly with limestone in the presence of water to form gypsum, which eventually flakes off or is dissolved by water. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. Thus we can write the reaction of limestone. Acid Rain Dissolve Marble.
From slidetodoc.com
Acid rain The formation of acid rain Acid Acid Rain Dissolve Marble Protective coatings, pollution control, and using more resistant materials can help mitigate the effects. Acid rain dissolves limestone, marble, cement and sandstone. Acid rain damages structures such as the taj mahal and thomas jefferson memorial. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. The dissolved aluminum. Acid Rain Dissolve Marble.
From en.wikipedia.org
Acid rain Wikipedia Acid Rain Dissolve Marble Acid rain damages structures such as the taj mahal and thomas jefferson memorial. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Acid rain dissolves limestone, marble, cement and sandstone. Protective coatings, pollution control, and using more resistant materials can help mitigate the effects. When sulfurous, sulfuric, and nitric acids in. Acid Rain Dissolve Marble.
From www.youtube.com
Marble Reacts with Acid YouTube Acid Rain Dissolve Marble Acid rain damages structures such as the taj mahal and thomas jefferson memorial. Thus we can write the reaction of limestone or marble with dilute sulfuric acid as follows: Sulfur dioxide, an acid rain precursor, can react directly with limestone in the presence of water to form gypsum, which eventually flakes off or is dissolved by water. Acid rain damages. Acid Rain Dissolve Marble.
From slideplayer.com
TOPIC IX WEATHERING AND EROSION ppt download Acid Rain Dissolve Marble The dissolved aluminum begins to accumulate and. Caco3(s) + h2so4(aq) → caso4(s) + h2o(l) + co2(g) (16.13.4) because caso 4 is. Acid rain damages buildings by corroding metals and dissolving stone, especially limestone and marble. Acid rain damages structures such as the taj mahal and thomas jefferson memorial. When sulfurous, sulfuric, and nitric acids in polluted air and rain react. Acid Rain Dissolve Marble.