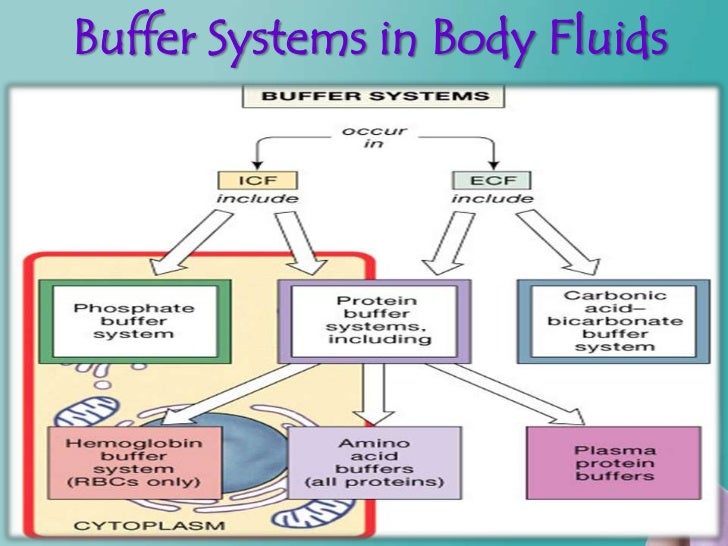
List Of Buffer Systems . Buffers contain a weak acid (\(ha\)) and its. Buffers are solutions that resist a change in ph after adding an acid or a base. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Good buffering systems have the following characteristics: The solution contains a weak acid and its conjugate base or a weak base and its conjugate acid the buffer resists changes in ph by. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer.
from www.slideshare.net
A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. It is able to neutralize small. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Buffers contain a weak acid (\(ha\)) and its. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are solutions that resist a change in ph after adding an acid or a base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The solution contains a weak acid and its conjugate base or a weak base and its conjugate acid the buffer resists changes in ph by. Good buffering systems have the following characteristics:
Buffer system
List Of Buffer Systems A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are solutions that resist a change in ph after adding an acid or a base. Buffers contain a weak acid (\(ha\)) and its. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. It is able to neutralize small. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Good buffering systems have the following characteristics: A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. The solution contains a weak acid and its conjugate base or a weak base and its conjugate acid the buffer resists changes in ph by.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance List Of Buffer Systems A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. A buffer is a solution that can resist ph change upon the addition of an. List Of Buffer Systems.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free List Of Buffer Systems A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. The solution contains a weak acid and its conjugate base or a weak base and its conjugate acid the. List Of Buffer Systems.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and List Of Buffer Systems A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are solutions that resist a change in ph after adding an acid or a base. It is able to neutralize small. Buffers contain a weak acid (\(ha\)) and its. The solution contains a weak acid and its. List Of Buffer Systems.
From quizlet.com
Bicarbonate Buffer system Diagram Quizlet List Of Buffer Systems A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. It is able to neutralize small. Buffers are solutions that resist a change in ph after adding an. List Of Buffer Systems.
From www.slideshare.net
Buffer system List Of Buffer Systems A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Buffers are solutions that resist a change in ph after adding an acid or a base. A buffer. List Of Buffer Systems.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID List Of Buffer Systems A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Good buffering systems have the following characteristics: The solution contains a weak acid and its conjugate base or a weak base and its conjugate acid the buffer resists changes in ph. List Of Buffer Systems.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide List Of Buffer Systems Good buffering systems have the following characteristics: A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers are solutions that resist. List Of Buffer Systems.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation List Of Buffer Systems Buffers are solutions that resist a change in ph after adding an acid or a base. Buffers contain a weak acid (\(ha\)) and its. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer is a solution that can. List Of Buffer Systems.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID List Of Buffer Systems Good buffering systems have the following characteristics: Buffers contain a weak acid (\(ha\)) and its. It is able to neutralize small. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. The solution contains a weak acid and its conjugate base or a weak base and its. List Of Buffer Systems.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 List Of Buffer Systems It is able to neutralize small. Buffers contain a weak acid (\(ha\)) and its. The solution contains a weak acid and its conjugate base or a weak base and its conjugate acid the buffer resists changes in ph by. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers are. List Of Buffer Systems.
From quizparaguayan.z4.web.core.windows.net
How Bicarbonate Buffer System Works List Of Buffer Systems It is able to neutralize small. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are solutions that resist a change in ph. List Of Buffer Systems.
From www.slideserve.com
PPT Chapter 13 AcidBase Balance PowerPoint Presentation, free List Of Buffer Systems It is able to neutralize small. The solution contains a weak acid and its conjugate base or a weak base and its conjugate acid the buffer resists changes in ph by. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Good buffering systems have the following characteristics: A mixture of. List Of Buffer Systems.
From www.slideserve.com
PPT Chapter 27 Fluid, Electrolyte and Acidbase Balance PowerPoint List Of Buffer Systems Buffers contain a weak acid (\(ha\)) and its. It is able to neutralize small. Good buffering systems have the following characteristics: A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer is a solution that maintains the stability of. List Of Buffer Systems.
From study.com
Buffer System in Chemistry Definition & Overview Video & Lesson List Of Buffer Systems A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers are solutions that resist a change in ph after adding an acid or a base. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers contain a. List Of Buffer Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint List Of Buffer Systems A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. The solution contains a weak acid and its conjugate base or a weak base and its conjugate acid the buffer resists changes in ph by. Buffers are solutions that resist a change in ph after adding an. List Of Buffer Systems.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation List Of Buffer Systems A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and. List Of Buffer Systems.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses List Of Buffer Systems The solution contains a weak acid and its conjugate base or a weak base and its conjugate acid the buffer resists changes in ph by. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. A buffer is a solution that maintains the stability of a system’s. List Of Buffer Systems.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download List Of Buffer Systems A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a. List Of Buffer Systems.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 List Of Buffer Systems Buffers are solutions that resist a change in ph after adding an acid or a base. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. It is able to neutralize small. A variety of buffering systems permits blood and other. List Of Buffer Systems.
From www.pinterest.com
chemical and physiological pH buffers Biochemistry notes, Nursing List Of Buffer Systems A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. The solution contains a weak acid and its conjugate base or a weak base and its conjugate acid the buffer resists changes in ph by. Good buffering systems have the following. List Of Buffer Systems.
From poorvi77.blogspot.com
Poorveez ParadyzMedimuseion major buffer systems in the body List Of Buffer Systems A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. The solution contains a weak acid and its conjugate base or a weak base and its conjugate acid the buffer resists changes in ph by. It is able to neutralize small. A buffer is a solution that. List Of Buffer Systems.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG List Of Buffer Systems A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. A buffer is a solution that can resist ph change upon the addition of an. List Of Buffer Systems.
From www.sliderbase.com
Buffers Presentation Chemistry List Of Buffer Systems A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are solutions that resist a change in ph. List Of Buffer Systems.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent List Of Buffer Systems A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers are solutions that resist a change in ph after adding an acid or a base. The solution contains. List Of Buffer Systems.
From www.slideserve.com
PPT AcidBase Balance I. PowerPoint Presentation, free download ID List Of Buffer Systems A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Good buffering systems have the following characteristics: It is able to neutralize small. A variety of buffering systems permits. List Of Buffer Systems.
From preclaboratories.com
pH and Buffers How Buffer Solutions Maintain pH Precision Laboratories List Of Buffer Systems Buffers are solutions that resist a change in ph after adding an acid or a base. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or. List Of Buffer Systems.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 List Of Buffer Systems Good buffering systems have the following characteristics: The solution contains a weak acid and its conjugate base or a weak base and its conjugate acid the buffer resists changes in ph by. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Buffers are solutions that resist. List Of Buffer Systems.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint List Of Buffer Systems A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Good buffering systems have the following characteristics: A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Buffers. List Of Buffer Systems.
From www.researchgate.net
List of buffers used in various experimentation. All buffers were List Of Buffer Systems A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Buffers are solutions that resist a change in ph after adding an acid or a base. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid). List Of Buffer Systems.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download List Of Buffer Systems Good buffering systems have the following characteristics: A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Buffers contain a weak acid (\(ha\)) and its. A buffer is a solution that can resist ph change upon the addition of an acidic. List Of Buffer Systems.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID List Of Buffer Systems A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Good buffering systems have the following characteristics: The solution contains a weak acid and its conjugate base or a weak base and its conjugate acid the buffer resists changes in ph by. A mixture of a weak acid. List Of Buffer Systems.
From www.slideshare.net
Buffer system List Of Buffer Systems A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The solution contains a weak acid and its conjugate base or a weak base and its conjugate acid the buffer resists changes in ph by. It is able to neutralize small. Buffers are solutions that resist a change in ph after. List Of Buffer Systems.
From www.studocu.com
Biological Buffer Systems BIOLOGICAL BUFFER SYSTEMS Almost every List Of Buffer Systems A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. Buffers contain a weak acid (\(ha\)) and its. Good buffering systems have the. List Of Buffer Systems.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint List Of Buffer Systems A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers contain a weak acid (\(ha\)) and its. Buffers are solutions that resist a change in ph after adding. List Of Buffer Systems.
From www.slideserve.com
PPT Acid, Base, Electrolytes PowerPoint Presentation, free download List Of Buffer Systems A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The solution contains a weak acid and its conjugate base or a. List Of Buffer Systems.