Zinc Sulfate Ph . Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. We need to identify an unknown for a chemistry lab. Learn about its definition, structure, preparation, properties, uses, and benefits. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. We know that zinc sulfate is acidic, but what is the ph of starch? Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. It's either starch or zinc sulfate. What is the ph of zinc sulfate? Uncover its role in health, agriculture, and industry! The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was measured over a range of.
from www.semanticscholar.org
Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was measured over a range of. Uncover its role in health, agriculture, and industry! What is the ph of zinc sulfate? The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc. We know that zinc sulfate is acidic, but what is the ph of starch? Learn about its definition, structure, preparation, properties, uses, and benefits. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. We need to identify an unknown for a chemistry lab.
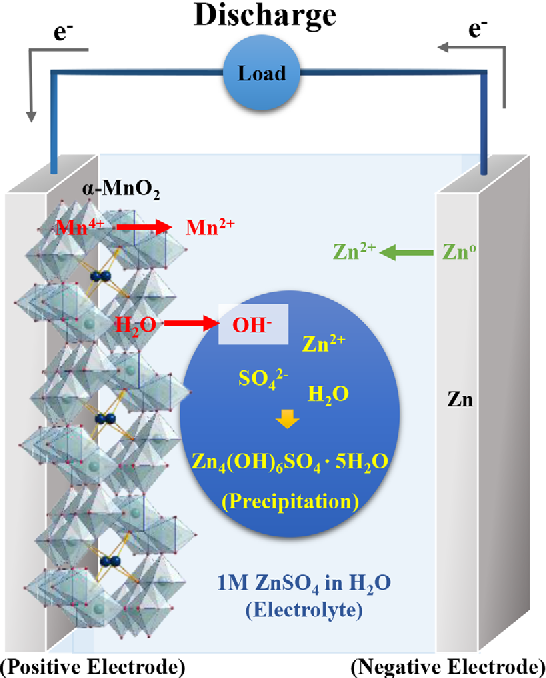
Figure 11 from Critical Role of pH Evolution of Electrolyte in the
Zinc Sulfate Ph What is the ph of zinc sulfate? We need to identify an unknown for a chemistry lab. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc. It's either starch or zinc sulfate. We know that zinc sulfate is acidic, but what is the ph of starch? The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was measured over a range of. Uncover its role in health, agriculture, and industry! Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. What is the ph of zinc sulfate? Learn about its definition, structure, preparation, properties, uses, and benefits.
From www.sustarfeed.com
China Zinc Sulfate Monohydrate ZnSO4 White Powder Animal Feed Additive Zinc Sulfate Ph The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. What. Zinc Sulfate Ph.
From www.pinterest.com
Zinc Sulphate Monohydrate Zinc sulfate, Sources of zinc, Zinc Zinc Sulfate Ph The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. Uncover its role in health, agriculture, and industry! We need to identify an unknown for a chemistry lab. We know that zinc sulfate is acidic, but what is the ph of starch? Zinc sulfate is an inorganic salt that dissolves in water to. Zinc Sulfate Ph.
From www.walmart.com
Zinc Sulfate 220 mg 100 Count Tablets Zinc Sulfate Ph What is the ph of zinc sulfate? We need to identify an unknown for a chemistry lab. Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. Uncover its role in health, agriculture, and. Zinc Sulfate Ph.
From www.oldbridgeminerals.com
Zinc Sulfate Monohydrate Feed & Fertilizer Grade Zinc Sulfate Ph The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc. The. Zinc Sulfate Ph.
From www.ridvanmau.com
Zinc Sulfate Monohydrate Obat Apa Fakta Penting Tentang Suplemen Zinc Sulfate Ph The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. We need to identify an unknown for a chemistry lab. It's either starch or zinc sulfate. Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. The ph of both synthetic zinc sulfate solutions of various compositions and commercial. Zinc Sulfate Ph.
From www.semanticscholar.org
Figure 1 from Effect of pH, concentration and temperature on copper and Zinc Sulfate Ph Uncover its role in health, agriculture, and industry! It's either starch or zinc sulfate. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was measured over a range of. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. The formation of zinc ions depends on. Zinc Sulfate Ph.
From www.metabolics.com
Zinc Sulfate Supplements Metabolics Zinc Sulfate Ph The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was measured over a range of. We know that zinc sulfate is acidic, but what is the ph of starch? We need to identify an unknown for a chemistry lab. Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions.. Zinc Sulfate Ph.
From www.pinterest.com
Zinc Sulfate Heptahydrate Equivalent 220 mg Qty 100 Capsules Zinc Sulfate Ph Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. What is the ph of zinc sulfate? Learn about its definition, structure, preparation, properties, uses, and benefits. Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. The ph of both synthetic zinc sulfate. Zinc Sulfate Ph.
From www.researchgate.net
Fig. 2 Distribution diagram for zinc species as a function of the Zinc Sulfate Ph The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was measured over a range of. It's either starch or zinc sulfate. We need to identify an unknown for a chemistry lab. We know that zinc sulfate. Zinc Sulfate Ph.
From www.semanticscholar.org
Figure 11 from Critical Role of pH Evolution of Electrolyte in the Zinc Sulfate Ph We need to identify an unknown for a chemistry lab. Uncover its role in health, agriculture, and industry! Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. Learn about its definition, structure, preparation, properties, uses, and benefits. What is the ph of zinc sulfate? Zinc sulfate is. Zinc Sulfate Ph.
From www.walmart.com
Zinc Sulfate 220 mg 100 Count Capsules Dietary Supplement Zinc Sulfate Ph We know that zinc sulfate is acidic, but what is the ph of starch? Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. It's either starch or zinc sulfate. Learn about its definition, structure, preparation, properties, uses, and benefits. What is the ph of zinc sulfate? The ph of both synthetic zinc sulfate solutions of. Zinc Sulfate Ph.
From fossilcreektreefarm.com
Hi Yield Zinc Sulfate 4 lb Fossil Creek Tree Farm Zinc Sulfate Ph The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. Uncover its role in health, agriculture, and industry! Zinc. Zinc Sulfate Ph.
From www.lazada.com.ph
ZINC VITA (Zinc Sulfate Oral Drops 15mL) Lazada PH Zinc Sulfate Ph Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was measured over a range of. We need to identify. Zinc Sulfate Ph.
From www.researchgate.net
Zn 2+ solubility diagram simulated by DIASTAB. Download Scientific Zinc Sulfate Ph Uncover its role in health, agriculture, and industry! It's either starch or zinc sulfate. Learn about its definition, structure, preparation, properties, uses, and benefits. What is the ph of zinc sulfate? Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. The formation of zinc ions depends on water ph and the presence of agents that. Zinc Sulfate Ph.
From www.lazada.com.ph
Zinc Sulfate (EZinc oral drops)15ml Lazada PH Zinc Sulfate Ph We know that zinc sulfate is acidic, but what is the ph of starch? It's either starch or zinc sulfate. Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. The formation of zinc ions depends on water ph and the presence of agents that can bind and. Zinc Sulfate Ph.
From www.unilab.com.ph
eZinc Children's Nutritional Supplement Unilab Zinc Sulfate Ph The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was measured over a range of. Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc. What is the ph. Zinc Sulfate Ph.
From beautybox.com.vn
Nước Xịt Khoáng Cho Da Dầu Mụn La RochePosay Serozinc Zinc Sulfate Zinc Sulfate Ph Learn about its definition, structure, preparation, properties, uses, and benefits. We need to identify an unknown for a chemistry lab. What is the ph of zinc sulfate? Uncover its role in health, agriculture, and industry! Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. Zinc sulfate is. Zinc Sulfate Ph.
From healthsynergy.co.za
Vimergy Organic Liquid Zinc Sulfate 60ml Synergy Zinc Sulfate Ph Learn about its definition, structure, preparation, properties, uses, and benefits. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was measured over a range of. We need to identify an unknown for a chemistry lab. It's either starch or zinc sulfate. What is the ph of zinc sulfate? Zinc sulfate is an inorganic. Zinc Sulfate Ph.
From shopee.ph
EZINC (Zinc Sulfate) SYRUP 55MG/5ML 60ML Shopee Philippines Zinc Sulfate Ph What is the ph of zinc sulfate? Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. Learn about its definition, structure, preparation, properties, uses, and benefits. Uncover its role in health, agriculture, and industry! It's either starch or zinc sulfate. The formation of zinc ions depends on. Zinc Sulfate Ph.
From www.walmart.com
Zinc Sulfate Monohydrate Powder 35.5 Zn 99.9 Pure 25 Lbs Zinc Sulfate Ph Uncover its role in health, agriculture, and industry! Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. What is the ph of zinc sulfate? We need to identify an unknown for a chemistry lab. We know that zinc sulfate is acidic, but what is the ph of. Zinc Sulfate Ph.
From www.lazada.co.id
Zinc sulfate 20mg strip 10 tab Lazada Indonesia Zinc Sulfate Ph Learn about its definition, structure, preparation, properties, uses, and benefits. Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. Uncover its role in health, agriculture, and industry! The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc.. Zinc Sulfate Ph.
From mgkb5oren.ru
Аллергия на сульфат цинка фото презентация Zinc Sulfate Ph We need to identify an unknown for a chemistry lab. Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. It's either starch or zinc sulfate. Learn about its definition, structure, preparation, properties, uses, and benefits. The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc.. Zinc Sulfate Ph.
From www.youtube.com
How to Write the Formula for Zinc sulfide (ZnS) YouTube Zinc Sulfate Ph The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc. Uncover its role in health, agriculture, and industry! What. Zinc Sulfate Ph.
From www.drugsupplystore.com
Zinc Sulfate Caplets 220mg 100ct Zinc Sulfate Ph Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was measured over a range of. The formation of zinc ions depends on water ph and the presence of agents that can. Zinc Sulfate Ph.
From www.nagwa.com
Vidéo question Identifier le résultat de l’ajout d’une solution de Zinc Sulfate Ph Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc. What is the ph. Zinc Sulfate Ph.
From zincsulfateco.com
ZINC SULPHATE MONOHYDRATE (ZnSO₄ . H2O) Zinc Sulfate Co. Zinc Sulfate Ph Uncover its role in health, agriculture, and industry! The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc. We know that zinc sulfate is acidic, but what is the ph of starch? It's either starch or zinc sulfate. Zinc sulfate is an inorganic salt that dissolves in water to form. Zinc Sulfate Ph.
From shopee.co.id
Jual ZINC SULFATE KIMIA FARMA 20MG 10 TABLET Shopee Indonesia Zinc Sulfate Ph What is the ph of zinc sulfate? Learn about its definition, structure, preparation, properties, uses, and benefits. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. We know that zinc sulfate is acidic, but what is the ph of starch? Uncover its role in health, agriculture, and industry! It's either starch or. Zinc Sulfate Ph.
From casida.com
Casida Zinc Drops ionic zinc sulfate pharmacy quality Casida® Zinc Sulfate Ph We need to identify an unknown for a chemistry lab. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc. Zinc sulfate is an inorganic salt that dissolves in water to form zinc. Zinc Sulfate Ph.
From fra.labbox.com
Zinc sulfate heptahydraté AGR, ACS, Ph.Eur. Labbox France Zinc Sulfate Ph The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. Learn about its definition, structure, preparation, properties, uses, and benefits. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was measured over. Zinc Sulfate Ph.
From www.johnsonfeedcompany.com
HiYield Zinc Sulfate Johnson Feed Company Zinc Sulfate Ph It's either starch or zinc sulfate. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was measured over a range of. What is the ph of zinc sulfate? The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. We know that zinc sulfate is acidic, but. Zinc Sulfate Ph.
From www.dfcsl.com
Zinc Sulfate Manufacturers Companies In India Zinc Sulfate Ph Uncover its role in health, agriculture, and industry! The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc. The ph of both synthetic zinc sulfate solutions of various compositions and commercial zinc plant electrolytes was. It's either starch or zinc sulfate. Learn about its definition, structure, preparation, properties, uses, and. Zinc Sulfate Ph.
From happyagri.com.vn
Phân Bón ZNSO4.H2O ZINC SULFATE MONOHYDRATE KIRNS 25KG Zinc Sulfate Ph Learn about its definition, structure, preparation, properties, uses, and benefits. The formation of zinc ions depends on water ph and the presence of agents that can bind and capture zinc. We know that zinc sulfate is acidic, but what is the ph of starch? Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. It's either. Zinc Sulfate Ph.
From www.grainger.com
RPI Zinc Sulfate/Monohydrate, Powder, 5 kg, 1 EA 31GF01Z223505000.0 Zinc Sulfate Ph What is the ph of zinc sulfate? It's either starch or zinc sulfate. Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. Uncover its role in health, agriculture, and industry! Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. The ph of. Zinc Sulfate Ph.
From www.amazon.com
Ionic Liquid Zinc Drops Zinc Sulfate for Immune Support Zinc Sulfate Ph It's either starch or zinc sulfate. We know that zinc sulfate is acidic, but what is the ph of starch? Learn about its definition, structure, preparation, properties, uses, and benefits. Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc. The ph of both synthetic zinc sulfate solutions. Zinc Sulfate Ph.
From www.indiamart.com
Zincate (Zinc Sulphate 21 ), For Fertilizer at best price in Gondia Zinc Sulfate Ph Zinc sulfate is an inorganic salt that dissolves in water to form zinc ions. We know that zinc sulfate is acidic, but what is the ph of starch? Learn about its definition, structure, preparation, properties, uses, and benefits. Zinc sulfate is about 36.5 percent elemental zinc, meaning that 220 mg of zinc sulfate would be about 50 mg of zinc.. Zinc Sulfate Ph.