Plain Language Labelling Regulations For Prescription Drugs . This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. This document provides information for industry on how health canada's health products and food branch interprets and. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014.
from www.fda.gov
The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. This document provides information for industry on how health canada's health products and food branch interprets and. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information.
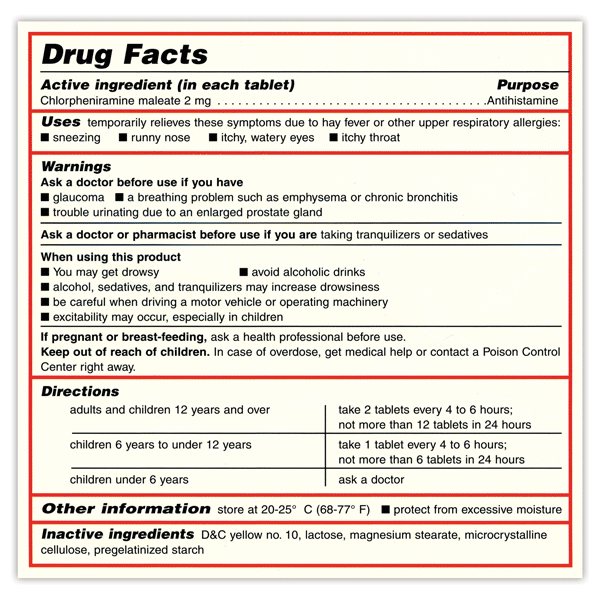
OTC Drug Facts Label FDA
Plain Language Labelling Regulations For Prescription Drugs This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. This document provides information for industry on how health canada's health products and food branch interprets and. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best.
From dicentra.com
Canada's Plain Language Labelling Regulations Compliance Plain Language Labelling Regulations For Prescription Drugs This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. This document. Plain Language Labelling Regulations For Prescription Drugs.
From www.healthywomen.org
How important is all the information on the labels of overthecounter Plain Language Labelling Regulations For Prescription Drugs The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. This guidance is intended to assist. Plain Language Labelling Regulations For Prescription Drugs.
From www.semanticscholar.org
Controlled Drugs Regulations and Prescribing Semantic Scholar Plain Language Labelling Regulations For Prescription Drugs The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. This guidance explains the legal. Plain Language Labelling Regulations For Prescription Drugs.
From www.studentdoctor.net
How to Write a Prescription 7 Steps for Safety SDN Plain Language Labelling Regulations For Prescription Drugs Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. The 2014 regulations amending. Plain Language Labelling Regulations For Prescription Drugs.
From www.pinterest.co.kr
Prescription Abbreviations Decoded common sig codes used in medical Plain Language Labelling Regulations For Prescription Drugs The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. This guidance is intended to assist applicants in. Plain Language Labelling Regulations For Prescription Drugs.
From www.fda.gov
OTC Drug Facts Label FDA Plain Language Labelling Regulations For Prescription Drugs This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. This document provides information for industry on how health canada's health products and food branch interprets and. The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. Medicines must include a. Plain Language Labelling Regulations For Prescription Drugs.
From bnf.nice.org.uk
Prescription writing Medicines guidance BNF NICE Plain Language Labelling Regulations For Prescription Drugs The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. This document provides information for. Plain Language Labelling Regulations For Prescription Drugs.
From dokumen.tips
(PDF) Plain Language Labelling & Health Canada Guidance on Look Plain Language Labelling Regulations For Prescription Drugs This document provides information for industry on how health canada's health products and food branch interprets and. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. This document provides information for. Plain Language Labelling Regulations For Prescription Drugs.
From study.com
How to Label Prescription Medication for Veterinary Patients Video Plain Language Labelling Regulations For Prescription Drugs This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. The european. Plain Language Labelling Regulations For Prescription Drugs.
From www.slideserve.com
PPT VETERINARY DRUG USE AND PRESCRIBING Chapter 5 PowerPoint Plain Language Labelling Regulations For Prescription Drugs This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. This document provides information for industry. Plain Language Labelling Regulations For Prescription Drugs.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Plain Language Labelling Regulations For Prescription Drugs This document provides information for industry on how health canada's health products and food branch interprets and. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. The european medicines. Plain Language Labelling Regulations For Prescription Drugs.
From www.npc-corp.ca
Plain Language Labelling and Your Opinion — Natural Products Consulting Plain Language Labelling Regulations For Prescription Drugs This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. This. Plain Language Labelling Regulations For Prescription Drugs.
From geekymedics.com
Prescribing in Primary Care FP10 Geeky Medics Plain Language Labelling Regulations For Prescription Drugs This document provides information for industry on how health canada's health products and food branch interprets and. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. This guidance is intended. Plain Language Labelling Regulations For Prescription Drugs.
From www.slideshare.net
Chapter05 Plain Language Labelling Regulations For Prescription Drugs Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. This document provides information for industry. Plain Language Labelling Regulations For Prescription Drugs.
From delltech.com
What is Plain Language Labelling Health Canada Dell Tech Plain Language Labelling Regulations For Prescription Drugs This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. This guidance is intended to assist. Plain Language Labelling Regulations For Prescription Drugs.
From delltech.com
Plain Language Labelling Regulations for NonPrescription Drugs Dell Tech Plain Language Labelling Regulations For Prescription Drugs This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. This document provides information for industry on how health canada's health products and food branch interprets and. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. This guidance is intended to assist applicants. Plain Language Labelling Regulations For Prescription Drugs.
From ihrmagazine.com
Labels to get plainer language with new regulation IHR Magazine Plain Language Labelling Regulations For Prescription Drugs This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. This document provides information for industry on how health canada's health products and food branch interprets and. Medicines must include a. Plain Language Labelling Regulations For Prescription Drugs.
From everythingmedschool.com
How To Write a Prescription (With Examples) Everything Med School Plain Language Labelling Regulations For Prescription Drugs Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. This document provides information. Plain Language Labelling Regulations For Prescription Drugs.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Plain Language Labelling Regulations For Prescription Drugs The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. This document provides information for industry on how health canada's health products and food branch interprets and. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. This guidance. Plain Language Labelling Regulations For Prescription Drugs.
From www.elabelinc.com
Pharmaceutical Label Printing • Custom Pharmaceutical & Medical Labels Plain Language Labelling Regulations For Prescription Drugs This document provides information for industry on how health canada's health products and food branch interprets and. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. This guidance explains. Plain Language Labelling Regulations For Prescription Drugs.
From hub.arkansasbluecross.com
Deciphering Your Prescription Medication Label Blueprint Plain Language Labelling Regulations For Prescription Drugs The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. Medicines must include. Plain Language Labelling Regulations For Prescription Drugs.
From regulatory.axsource.com
Drug Labelling AXSource Plain Language Labelling Regulations For Prescription Drugs The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. This guidance is. Plain Language Labelling Regulations For Prescription Drugs.
From www.youtube.com
How to read a medication label YouTube Plain Language Labelling Regulations For Prescription Drugs The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. The 2014 regulations amending the food. Plain Language Labelling Regulations For Prescription Drugs.
From www.slideserve.com
PPT Handling of Medication PowerPoint Presentation, free download Plain Language Labelling Regulations For Prescription Drugs This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. This document provides information for industry on how health canada's health products and food branch interprets and. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. This guidance explains the legal. Plain Language Labelling Regulations For Prescription Drugs.
From my.clevelandclinic.org
How To Read A Prescription Label A Guide Cleveland Clinic Plain Language Labelling Regulations For Prescription Drugs This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. This guidance. Plain Language Labelling Regulations For Prescription Drugs.
From qualitysmartsolutions.com
Plain Language Labelling Regulations Quality Smart Solutions Plain Language Labelling Regulations For Prescription Drugs This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. Medicines. Plain Language Labelling Regulations For Prescription Drugs.
From delltech.com
Impact of Plain Language Labelling on Natural Health Products Dell Tech Plain Language Labelling Regulations For Prescription Drugs This document provides information for industry on how health canada's health products and food branch interprets and. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. This document provides information for industry on. Plain Language Labelling Regulations For Prescription Drugs.
From dokumen.tips
(PDF) Labels and Packages Certification Form for Prescription Plain Language Labelling Regulations For Prescription Drugs This document provides information for industry on how health canada's health products and food branch interprets and. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. The european medicines agency (ema). Plain Language Labelling Regulations For Prescription Drugs.
From ar.inspiredpencil.com
Prescription Plain Language Labelling Regulations For Prescription Drugs This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. This document provides information for industry on how health canada's health products and food branch interprets and. This guidance is intended to assist applicants in complying. Plain Language Labelling Regulations For Prescription Drugs.
From www.cbc.ca
Hardtoread drug labels could lead to dosage errors CBC News Plain Language Labelling Regulations For Prescription Drugs This document provides information for industry on how health canada's health products and food branch interprets and. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. The 2014 regulations amending the. Plain Language Labelling Regulations For Prescription Drugs.
From mediqueproducts.com
Medique Products The Brands That Work Plain Language Labelling Regulations For Prescription Drugs This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The european medicines agency (ema) makes guidance and templates available to provide marketing authorisation applicants with. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. This document provides information for industry. Plain Language Labelling Regulations For Prescription Drugs.
From www.tataelxsi.com
Tata Elxsi The New Plain Language Labeling (PLL) Regulations by Plain Language Labelling Regulations For Prescription Drugs This document provides information for industry on how health canada's health products and food branch interprets and. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. The european medicines agency (ema) makes guidance. Plain Language Labelling Regulations For Prescription Drugs.
From dokumen.tips
(PDF) Plain Language Labelling (PLL) Requirements DOKUMEN.TIPS Plain Language Labelling Regulations For Prescription Drugs This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. Medicines must include a patient information leaflet (pil) if the label does not contain all the necessary information. This document provides. Plain Language Labelling Regulations For Prescription Drugs.
From www.languageconnections.com
Do You Understand Your Prescription? Language Connections Blog Plain Language Labelling Regulations For Prescription Drugs This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. This document provides information for industry on how health canada's health products and food branch interprets and. The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. This guidance is intended. Plain Language Labelling Regulations For Prescription Drugs.
From financialpost.com
Christelle Gedeon Plain language drug labelling project is a sight for Plain Language Labelling Regulations For Prescription Drugs This document provides information for industry on how health canada's health products and food branch interprets and. This document provides information for industry on how health canada’s health products and food branch interprets and applies the 2014. The 2014 regulations amending the food and drug regulations (labelling, packaging and brand names of drugs for human use) are commonly. This guidance. Plain Language Labelling Regulations For Prescription Drugs.