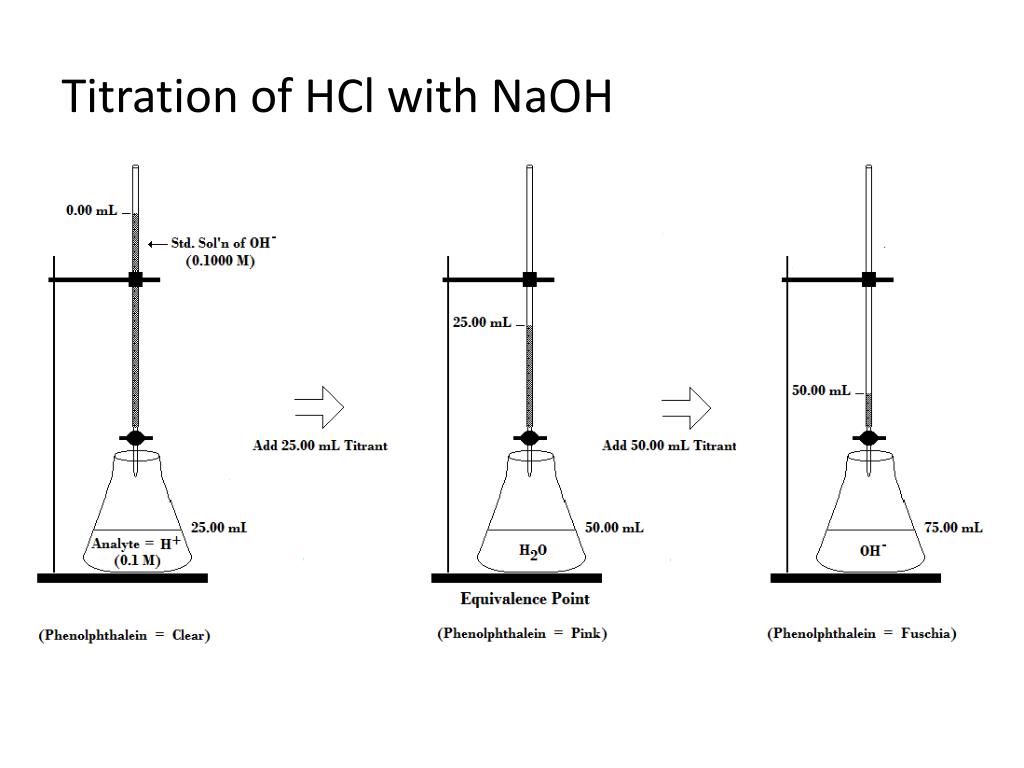
Titration Of Hcl And Naoh Calculation . They then concentrate the solution and allow it to. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. Concordant titres should be used when. Data collected during a titration allows chemists to determine a solution’s unknown concentration. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Multiply the molarity of the strong base naoh by the volume of. First determine the moles of \(\ce{naoh}\) in the reaction. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as:
from www.vrogue.co
Concordant titres should be used when. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Multiply the molarity of the strong base naoh by the volume of. They then concentrate the solution and allow it to. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. First determine the moles of \(\ce{naoh}\) in the reaction.
Titration Practical And Calculation Naoh And Hcl Yout vrogue.co
Titration Of Hcl And Naoh Calculation In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. Data collected during a titration allows chemists to determine a solution’s unknown concentration. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. Concordant titres should be used when. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. First determine the moles of \(\ce{naoh}\) in the reaction. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Multiply the molarity of the strong base naoh by the volume of.
From mungfali.com
HCl And NaOH Equation Titration Of Hcl And Naoh Calculation Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known. Titration Of Hcl And Naoh Calculation.
From www.slideserve.com
PPT Reactions in Aqueous Solutions Calculations PowerPoint Titration Of Hcl And Naoh Calculation From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Data collected during a titration allows chemists to determine a solution’s unknown concentration. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. A titration is used to find an unknown concentration of a solution by reacting it with a. Titration Of Hcl And Naoh Calculation.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Titration Of Hcl And Naoh Calculation From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Multiply the molarity of the strong base naoh by the volume of. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Concordant titres should. Titration Of Hcl And Naoh Calculation.
From studylib.net
Titration of HCl with NaOH Titration Of Hcl And Naoh Calculation First determine the moles of \(\ce{naoh}\) in the reaction. Concordant titres should be used when. They then concentrate the solution and allow it to. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by. Titration Of Hcl And Naoh Calculation.
From www.gulfsouthsolar.com
Extraordinario Solenoide Formación titration of sodium hydroxide with Titration Of Hcl And Naoh Calculation In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. A titration is used to find an unknown concentration of a solution by reacting it with. Titration Of Hcl And Naoh Calculation.
From www.youtube.com
Lab 2 (Titration of HCl with Na2CO3) YouTube Titration Of Hcl And Naoh Calculation Concordant titres should be used when. Multiply the molarity of the strong base naoh by the volume of. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration.. Titration Of Hcl And Naoh Calculation.
From www.vrogue.co
Titration Practical And Calculation Naoh And Hcl Yout vrogue.co Titration Of Hcl And Naoh Calculation In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Concordant titres should be used when. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution. Titration Of Hcl And Naoh Calculation.
From www.vrogue.co
What Is The Chemical Equation For Titration Of Hcl Nh vrogue.co Titration Of Hcl And Naoh Calculation From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. Concordant titres should be used when. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite. Titration Of Hcl And Naoh Calculation.
From mungfali.com
HCl NaOH Titration Titration Of Hcl And Naoh Calculation Concordant titres should be used when. Data collected during a titration allows chemists to determine a solution’s unknown concentration. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. First determine the moles of \(\ce{naoh}\) in the reaction. A titration is used to find an. Titration Of Hcl And Naoh Calculation.
From mungfali.com
HCl NaOH Titration Titration Of Hcl And Naoh Calculation First determine the moles of \(\ce{naoh}\) in the reaction. Concordant titres should be used when. Data collected during a titration allows chemists to determine a solution’s unknown concentration. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. They then concentrate the solution and allow it to. Multiply the molarity of the strong base naoh by the volume of.. Titration Of Hcl And Naoh Calculation.
From www.numerade.com
SOLVED In a titration of hydrochloric acid against sodium hydroxide Titration Of Hcl And Naoh Calculation Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: They then concentrate the solution and allow it to. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. Concordant titres should be used. Titration Of Hcl And Naoh Calculation.
From slideplayer.com
Titration Calculation ppt download Titration Of Hcl And Naoh Calculation They then concentrate the solution and allow it to. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Multiply the molarity of the strong base naoh by the volume of. First determine the moles of \(\ce{naoh}\) in the reaction. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by. Titration Of Hcl And Naoh Calculation.
From mungfali.com
HCl NaOH Titration Titration Of Hcl And Naoh Calculation In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to. Multiply the molarity of the strong base naoh by the volume of. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known. Titration Of Hcl And Naoh Calculation.
From mungfali.com
HCl NaOH Titration Titration Of Hcl And Naoh Calculation They then concentrate the solution and allow it to. Multiply the molarity of the strong base naoh by the volume of. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. Concordant titres should be used when. In this experiment students neutralise sodium hydroxide with. Titration Of Hcl And Naoh Calculation.
From www.studypool.com
SOLUTION Rvs chemistry 11 hydrochloric acid and sodium hydroxide Titration Of Hcl And Naoh Calculation Concordant titres should be used when. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Data collected during a titration allows chemists to determine a solution’s unknown concentration. Multiply the molarity of the strong base naoh by the volume of. In this experiment students neutralise sodium hydroxide with. Titration Of Hcl And Naoh Calculation.
From www.numerade.com
SOLVED Write the chemical equation for the titration of HCl and NaOH Titration Of Hcl And Naoh Calculation Concordant titres should be used when. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Multiply the molarity of the strong base naoh by the volume of.. Titration Of Hcl And Naoh Calculation.
From mungfali.com
HCl NaOH Titration Titration Of Hcl And Naoh Calculation Concordant titres should be used when. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: A titration is used to find an unknown concentration. Titration Of Hcl And Naoh Calculation.
From www.vrogue.co
What Is The Chemical Equation For Titration Of Hcl Nh vrogue.co Titration Of Hcl And Naoh Calculation Multiply the molarity of the strong base naoh by the volume of. Data collected during a titration allows chemists to determine a solution’s unknown concentration. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly. Titration Of Hcl And Naoh Calculation.
From www.vrogue.co
Titration Practical And Calculation Naoh And Hcl Yout vrogue.co Titration Of Hcl And Naoh Calculation They then concentrate the solution and allow it to. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. In a titration, 25.00 cm 3 of 0.200 mol/dm. Titration Of Hcl And Naoh Calculation.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Titration Of Hcl And Naoh Calculation They then concentrate the solution and allow it to. Multiply the molarity of the strong base naoh by the volume of. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3. Titration Of Hcl And Naoh Calculation.
From www.learnsci.com
LearnSci Smart Worksheet Determine Concentration of HCl by Titration Titration Of Hcl And Naoh Calculation A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Multiply the molarity of the strong base naoh by the volume of. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium. Titration Of Hcl And Naoh Calculation.
From www.youtube.com
Titration of HCl with NaOH YouTube Titration Of Hcl And Naoh Calculation Multiply the molarity of the strong base naoh by the volume of. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Concordant titres should be used when. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration.. Titration Of Hcl And Naoh Calculation.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Titration Of Hcl And Naoh Calculation They then concentrate the solution and allow it to. Multiply the molarity of the strong base naoh by the volume of. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. In a titration, 25.00 cm 3 of 0.200 mol/dm. Titration Of Hcl And Naoh Calculation.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Titration Of Hcl And Naoh Calculation Multiply the molarity of the strong base naoh by the volume of. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. In a titration, 25.00 cm 3. Titration Of Hcl And Naoh Calculation.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Of Hcl And Naoh Calculation Data collected during a titration allows chemists to determine a solution’s unknown concentration. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. First determine the moles of \(\ce{naoh}\) in the reaction. Multiply the molarity of the strong base naoh by the volume of. In a titration, 25.00 cm. Titration Of Hcl And Naoh Calculation.
From mungfali.com
HCl NaOH Titration Titration Of Hcl And Naoh Calculation A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. First determine the. Titration Of Hcl And Naoh Calculation.
From www.numerade.com
SOLVED The following data were collected during a titration Calculate Titration Of Hcl And Naoh Calculation Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: First determine the moles of \(\ce{naoh}\) in the reaction. Multiply the molarity of the strong base naoh by the volume of. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70. Titration Of Hcl And Naoh Calculation.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Titration Of Hcl And Naoh Calculation Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: First determine the moles of \(\ce{naoh}\) in the reaction. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. A titration is used to find an unknown concentration of a. Titration Of Hcl And Naoh Calculation.
From www.youtube.com
Titration of (NaOH+Na2CO3) vs HCl with Calculation of Strength, gm/lt Titration Of Hcl And Naoh Calculation Multiply the molarity of the strong base naoh by the volume of. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised. Titration Of Hcl And Naoh Calculation.
From www.gulfsouthsolar.com
Extraordinario Solenoide Formación titration of sodium hydroxide with Titration Of Hcl And Naoh Calculation They then concentrate the solution and allow it to. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the. Titration Of Hcl And Naoh Calculation.
From www.numerade.com
SOLVED Calculate the pH in the titration of 10.00mL of 1.0M HCl with 1 Titration Of Hcl And Naoh Calculation They then concentrate the solution and allow it to. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. Concordant titres should be used when. Since. Titration Of Hcl And Naoh Calculation.
From www.vrogue.co
Titration Practical And Calculation Naoh And Hcl Yout vrogue.co Titration Of Hcl And Naoh Calculation They then concentrate the solution and allow it to. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. First determine the moles of \(\ce{naoh}\) in the reaction. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. In this experiment students neutralise sodium. Titration Of Hcl And Naoh Calculation.
From mungfali.com
Acid Base Titration Calculation Titration Of Hcl And Naoh Calculation They then concentrate the solution and allow it to. Concordant titres should be used when. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: First determine the moles of \(\ce{naoh}\) in the reaction. Data collected during a titration allows chemists to determine a solution’s unknown concentration. A titration. Titration Of Hcl And Naoh Calculation.
From exotnsiwz.blob.core.windows.net
Titration Of Naoh With Hcl at Katherine Grassi blog Titration Of Hcl And Naoh Calculation In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. Concordant titres should be used when. Since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Multiply the molarity of the strong base naoh. Titration Of Hcl And Naoh Calculation.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Titration Of Hcl And Naoh Calculation Data collected during a titration allows chemists to determine a solution’s unknown concentration. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. In this experiment students neutralise sodium hydroxide with hydrochloric acid to. Titration Of Hcl And Naoh Calculation.