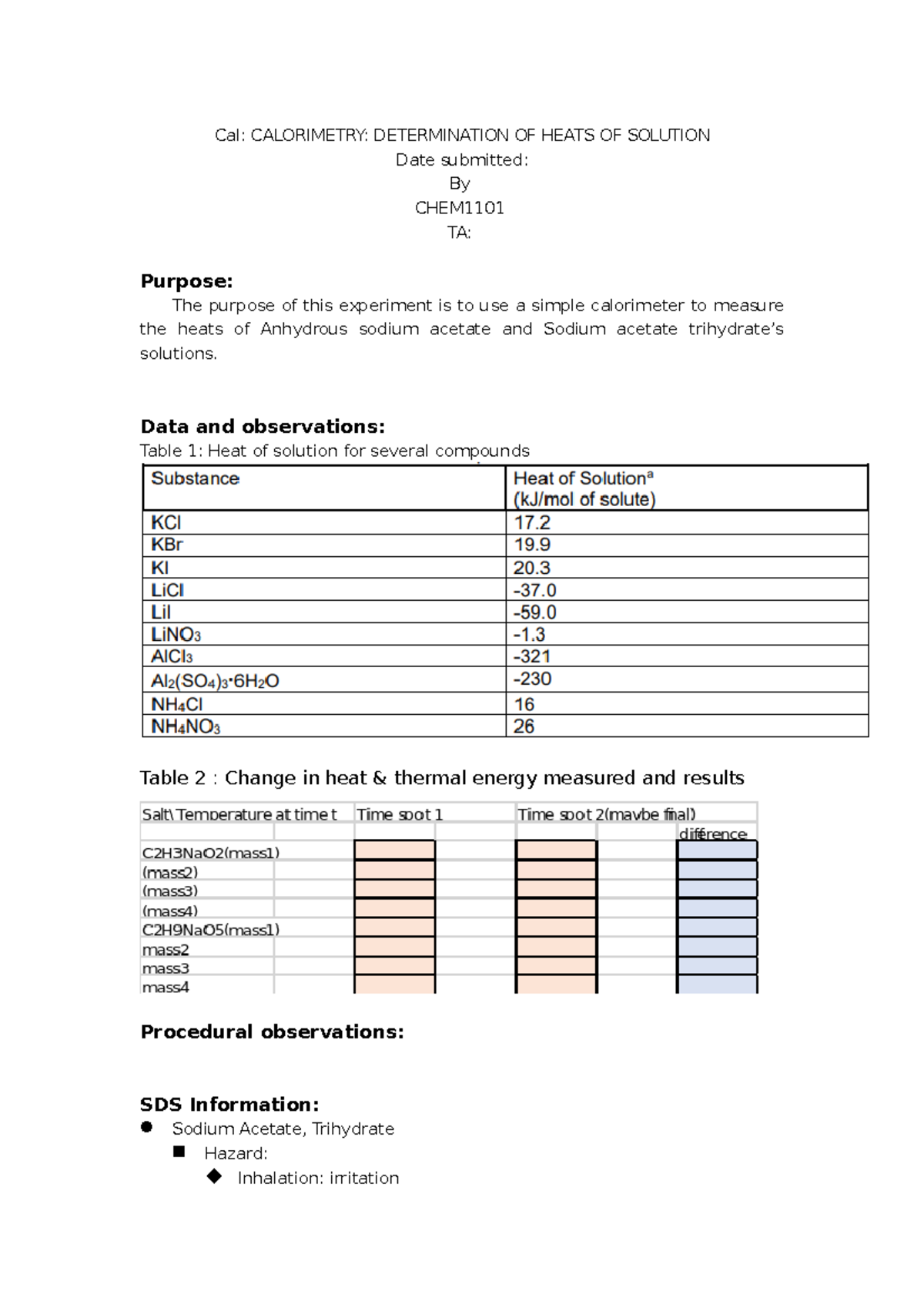
Calorimetry Determination Of Heats Of Solution Lab Report . Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of The document outlines a calorimetry experiment to determine the heats of solution for sodium acetate salts. Heat of solution of anhydrous nac 2 h 3 o 2 the calorimeter and thermometer must be dried with a clean tissue. This is a lab report for cal chem 1101. View cal lab report.docx from phys 1003 at carleton university. Determine specific heats (or heats of chemical reactions) is called a calorimeter. In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. This document details an experiment on calorimetry to determine the heats of solution for sodium acetate forms. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. Average molar heat of solution for sodium acetate trihydrate is.
from www.studocu.com
In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. Average molar heat of solution for sodium acetate trihydrate is. View cal lab report.docx from phys 1003 at carleton university. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. The document outlines a calorimetry experiment to determine the heats of solution for sodium acetate salts. This document details an experiment on calorimetry to determine the heats of solution for sodium acetate forms. Heat of solution of anhydrous nac 2 h 3 o 2 the calorimeter and thermometer must be dried with a clean tissue. This is a lab report for cal chem 1101. Determine specific heats (or heats of chemical reactions) is called a calorimeter. Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of
Cal prelab Cal CALORIMETRY DETERMINATION OF HEATS OF SOLUTION Date
Calorimetry Determination Of Heats Of Solution Lab Report This is a lab report for cal chem 1101. Average molar heat of solution for sodium acetate trihydrate is. Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of Determine specific heats (or heats of chemical reactions) is called a calorimeter. The document outlines a calorimetry experiment to determine the heats of solution for sodium acetate salts. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. This is a lab report for cal chem 1101. View cal lab report.docx from phys 1003 at carleton university. This document details an experiment on calorimetry to determine the heats of solution for sodium acetate forms. Heat of solution of anhydrous nac 2 h 3 o 2 the calorimeter and thermometer must be dried with a clean tissue. In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water.
From www.studocu.com
Cal Lab Report1 Lab report for lab 3 Calorimetry Determination of Calorimetry Determination Of Heats Of Solution Lab Report In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. View cal lab report.docx from phys 1003 at carleton university. Determine specific heats (or heats of chemical reactions) is called a calorimeter. Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.scribd.com
Lab 07Specific Heat & Calorimetry.pdf Heat Temperature Calorimetry Determination Of Heats Of Solution Lab Report Average molar heat of solution for sodium acetate trihydrate is. In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. This document details an experiment on calorimetry to determine the heats of solution for sodium acetate forms. Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose. Calorimetry Determination Of Heats Of Solution Lab Report.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimetry Determination Of Heats Of Solution Lab Report Average molar heat of solution for sodium acetate trihydrate is. This document details an experiment on calorimetry to determine the heats of solution for sodium acetate forms. This is a lab report for cal chem 1101. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. Determine specific heats (or heats. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Calorimetry Determination of Heats of Solution lab 4 Proceduce Calorimetry Determination Of Heats Of Solution Lab Report Determine specific heats (or heats of chemical reactions) is called a calorimeter. Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of The document outlines a calorimetry experiment to determine the heats of solution for sodium acetate salts. View cal lab report.docx from phys 1003 at carleton university. A perfect calorimeter absorbs no. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Cal Lab Short Report 20231124 Determination of Heats of Solution by Calorimetry Determination Of Heats Of Solution Lab Report Average molar heat of solution for sodium acetate trihydrate is. In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. View cal lab report.docx from phys 1003 at carleton university. Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of This is. Calorimetry Determination Of Heats Of Solution Lab Report.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Calorimetry Determination Of Heats Of Solution Lab Report Determine specific heats (or heats of chemical reactions) is called a calorimeter. This document details an experiment on calorimetry to determine the heats of solution for sodium acetate forms. Average molar heat of solution for sodium acetate trihydrate is. This is a lab report for cal chem 1101. The document outlines a calorimetry experiment to determine the heats of solution. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Cal prelab Cal CALORIMETRY DETERMINATION OF HEATS OF SOLUTION Date Calorimetry Determination Of Heats Of Solution Lab Report Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of Determine specific heats (or heats of chemical reactions) is called a calorimeter. Average molar heat of solution for sodium acetate trihydrate is. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. View cal. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Calorimetry LAb calomrimetry Calorimetry Determination of Heats of Calorimetry Determination Of Heats Of Solution Lab Report Determine specific heats (or heats of chemical reactions) is called a calorimeter. View cal lab report.docx from phys 1003 at carleton university. Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water.. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Cal Lab CAL Lab report Calorimetry Determination of Heats of Calorimetry Determination Of Heats Of Solution Lab Report Average molar heat of solution for sodium acetate trihydrate is. Heat of solution of anhydrous nac 2 h 3 o 2 the calorimeter and thermometer must be dried with a clean tissue. In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. View cal lab report.docx from phys 1003. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Cal prelab 01232023 18333430 CALORIMETRY DETERMINATION OF HEATS Calorimetry Determination Of Heats Of Solution Lab Report This document details an experiment on calorimetry to determine the heats of solution for sodium acetate forms. Heat of solution of anhydrous nac 2 h 3 o 2 the calorimeter and thermometer must be dried with a clean tissue. Determine specific heats (or heats of chemical reactions) is called a calorimeter. Determination of heats of solution dena chem 1001 a3. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Cal Write Up Lab report Calorimetry Determination of Heats of Calorimetry Determination Of Heats Of Solution Lab Report View cal lab report.docx from phys 1003 at carleton university. Heat of solution of anhydrous nac 2 h 3 o 2 the calorimeter and thermometer must be dried with a clean tissue. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. Determination of heats of solution dena chem 1001 a3. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Cal pre lab report Experiment 3 CALORIMETRY DETERMINATION OF HEATS Calorimetry Determination Of Heats Of Solution Lab Report Determine specific heats (or heats of chemical reactions) is called a calorimeter. Average molar heat of solution for sodium acetate trihydrate is. The document outlines a calorimetry experiment to determine the heats of solution for sodium acetate salts. Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of View cal lab report.docx from. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Cal PreLab Winter 2024 Calorimetry Determination of Heats of Calorimetry Determination Of Heats Of Solution Lab Report Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of The document outlines a calorimetry experiment to determine the heats of solution for sodium acetate salts. Heat of solution of anhydrous nac 2 h 3 o 2 the calorimeter and thermometer must be dried with a clean tissue. View cal lab report.docx from. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Lab report 1 calorimetry Calorimetry Determination of Heats of Calorimetry Determination Of Heats Of Solution Lab Report In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. The document outlines a calorimetry experiment to determine the heats of solution for sodium acetate salts. Determine specific heats (or heats of chemical reactions) is called a calorimeter. This is a lab report for cal chem 1101. Heat of. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
P calorimetry 25 lab report StuDocu Calorimetry Determination Of Heats Of Solution Lab Report Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of A perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. The document outlines a calorimetry experiment to determine the heats of solution for sodium acetate salts. Heat of solution of anhydrous nac 2 h. Calorimetry Determination Of Heats Of Solution Lab Report.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Calorimetry Determination Of Heats Of Solution Lab Report A perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. Average molar heat of solution for sodium acetate trihydrate is. Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of In this experiment, students will determine the amount of heat involved when ammonium chloride. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Lab short report Cal2 CALORIMETRY DETERMINATION OF HEATS OF Calorimetry Determination Of Heats Of Solution Lab Report Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of Heat of solution of anhydrous nac 2 h 3 o 2 the calorimeter and thermometer must be dried with a clean tissue. This is a lab report for cal chem 1101. A perfect calorimeter absorbs no heat from the solution that it contains,. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Cal prelab 01232023 Calorimetry Determination of Heats of Calorimetry Determination Of Heats Of Solution Lab Report Determine specific heats (or heats of chemical reactions) is called a calorimeter. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. Average molar heat of solution for sodium acetate trihydrate is. View cal lab report.docx from phys 1003 at carleton university. The document outlines a calorimetry experiment to determine the. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Calorimetry Prelab PreLab 1 Calorimetry Determination of Heats of Calorimetry Determination Of Heats Of Solution Lab Report Heat of solution of anhydrous nac 2 h 3 o 2 the calorimeter and thermometer must be dried with a clean tissue. Average molar heat of solution for sodium acetate trihydrate is. In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. A perfect calorimeter absorbs no heat from. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Calorimetry/Cal prelab template Calorimetry Determination of Heats Calorimetry Determination Of Heats Of Solution Lab Report This document details an experiment on calorimetry to determine the heats of solution for sodium acetate forms. The document outlines a calorimetry experiment to determine the heats of solution for sodium acetate salts. Average molar heat of solution for sodium acetate trihydrate is. View cal lab report.docx from phys 1003 at carleton university. In this experiment, students will determine the. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Cal PreLab CHEM 1002 Cal pre lab report CALORIMETRY DETERMINATION Calorimetry Determination Of Heats Of Solution Lab Report Determine specific heats (or heats of chemical reactions) is called a calorimeter. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. View cal lab report.docx from phys 1003 at carleton university. This is a lab report for cal chem 1101. Determination of heats of solution dena chem 1001 a3 kupa. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Determination Of Heats Of Solution Lab Report In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of This document details an experiment on calorimetry to determine the heats of solution for sodium acetate forms. This is a lab report. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Lab Manual Calorimetry Cal CALORIMETRY DETERMINATION OF HEATS OF Calorimetry Determination Of Heats Of Solution Lab Report Heat of solution of anhydrous nac 2 h 3 o 2 the calorimeter and thermometer must be dried with a clean tissue. In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. View cal lab report.docx from phys 1003 at carleton university. The document outlines a calorimetry experiment to. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimetry Determination Of Heats Of Solution Lab Report Average molar heat of solution for sodium acetate trihydrate is. In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. Determine specific heats (or heats of chemical reactions) is called a calorimeter. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Calorimetry Prelab CALORIMETRY DETERMINATION OF HEATS OF SOLUTION Calorimetry Determination Of Heats Of Solution Lab Report View cal lab report.docx from phys 1003 at carleton university. In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of Heat of solution of anhydrous nac 2 h 3 o 2 the. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Cal short report Calorimetry Determination of Heats of Solution Calorimetry Determination Of Heats Of Solution Lab Report Average molar heat of solution for sodium acetate trihydrate is. Heat of solution of anhydrous nac 2 h 3 o 2 the calorimeter and thermometer must be dried with a clean tissue. The document outlines a calorimetry experiment to determine the heats of solution for sodium acetate salts. Determine specific heats (or heats of chemical reactions) is called a calorimeter.. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Calorimetry Lab Calorimetry Lab Data and Results Table 1 Calorimetry Determination Of Heats Of Solution Lab Report This document details an experiment on calorimetry to determine the heats of solution for sodium acetate forms. The document outlines a calorimetry experiment to determine the heats of solution for sodium acetate salts. This is a lab report for cal chem 1101. In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
CalPreLab chem 1002 Calorimetry Determination of Heats of Solutions Calorimetry Determination Of Heats Of Solution Lab Report View cal lab report.docx from phys 1003 at carleton university. Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. This is a lab report for cal chem 1101. Average molar heat. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Determination Of Heats Of Solution Lab Report Average molar heat of solution for sodium acetate trihydrate is. This is a lab report for cal chem 1101. View cal lab report.docx from phys 1003 at carleton university. Heat of solution of anhydrous nac 2 h 3 o 2 the calorimeter and thermometer must be dried with a clean tissue. Determine specific heats (or heats of chemical reactions) is. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
CALORIMETRY DETERMINATION OF HEATS OF SOLUTION CALORIMETRY Calorimetry Determination Of Heats Of Solution Lab Report In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. This is a lab report for cal chem 1101. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. Determine specific heats (or heats of chemical reactions) is called a. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Prel Lab Cal, TA Kailai Wang, 03152023 Calorimetry Determination Calorimetry Determination Of Heats Of Solution Lab Report Average molar heat of solution for sodium acetate trihydrate is. Heat of solution of anhydrous nac 2 h 3 o 2 the calorimeter and thermometer must be dried with a clean tissue. This document details an experiment on calorimetry to determine the heats of solution for sodium acetate forms. Determine specific heats (or heats of chemical reactions) is called a. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Determination Of Heats Of Solution Lab Report Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose the measurements of heat of In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. This is a lab report for cal chem 1101. This document details an experiment on calorimetry to determine the heats of solution. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Cal pre lab Calorimetry pre lab Calorimetry Determination of Heats Calorimetry Determination Of Heats Of Solution Lab Report Heat of solution of anhydrous nac 2 h 3 o 2 the calorimeter and thermometer must be dried with a clean tissue. This document details an experiment on calorimetry to determine the heats of solution for sodium acetate forms. This is a lab report for cal chem 1101. Determination of heats of solution dena chem 1001 a3 kupa kupakuwana purpose. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Calorimetry Determination of Heats of Solution (Cal)5 Chem 1101 Calorimetry Determination Of Heats Of Solution Lab Report The document outlines a calorimetry experiment to determine the heats of solution for sodium acetate salts. In this experiment, students will determine the amount of heat involved when ammonium chloride (or calcium chloride) is dissolved in water. Determine specific heats (or heats of chemical reactions) is called a calorimeter. Average molar heat of solution for sodium acetate trihydrate is. Heat. Calorimetry Determination Of Heats Of Solution Lab Report.
From www.studocu.com
Calorimetry prelab pre lab Calorimetry Determination of heats of Calorimetry Determination Of Heats Of Solution Lab Report View cal lab report.docx from phys 1003 at carleton university. The document outlines a calorimetry experiment to determine the heats of solution for sodium acetate salts. This is a lab report for cal chem 1101. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. Determination of heats of solution dena. Calorimetry Determination Of Heats Of Solution Lab Report.