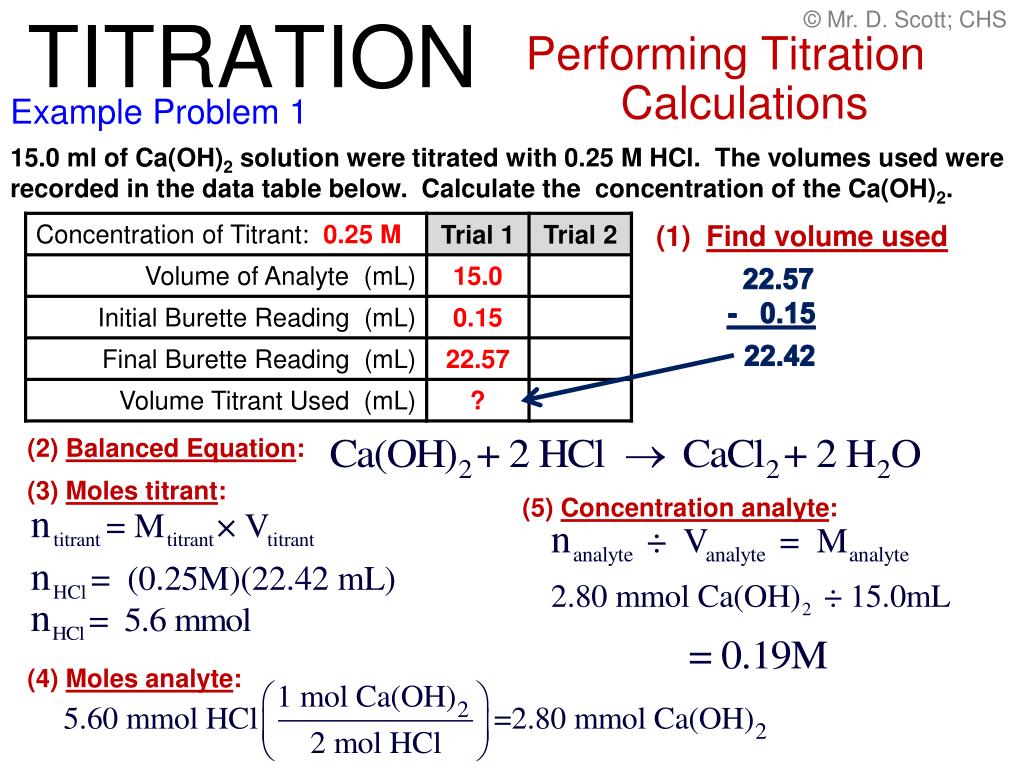
Titration Problems Equation . calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. A titration experiment aims to determine the. this webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. Titration of a weak acid with a strong base. this video walks through common examples of calculation problems for titration. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml.
from www.slideserve.com
this webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: this video walks through common examples of calculation problems for titration. titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). Titration of a weak acid with a strong base. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. A titration experiment aims to determine the. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the.
PPT TITRATION PowerPoint Presentation, free download ID1459481
Titration Problems Equation this video walks through common examples of calculation problems for titration. Titration of a weak acid with a strong base. A titration experiment aims to determine the. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. this webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. this video walks through common examples of calculation problems for titration.
From learningzonelisannin.z14.web.core.windows.net
Chemistry Titration Questions And Answers Titration Problems Equation A titration experiment aims to determine the. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a. Titration Problems Equation.
From www.slideserve.com
PPT Neutralization & Titration PowerPoint Presentation, free download Titration Problems Equation A titration experiment aims to determine the. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: a titration is a volumetric technique in which a solution of one. Titration Problems Equation.
From www.chegg.com
Solved a. Write the chemical equation for the titration Titration Problems Equation titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Titration Problems Equation.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Titration Problems Equation calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a. Titration Problems Equation.
From www.youtube.com
Titration Calculations GCSE Science grade 7, 8 and 9 Booster Titration Problems Equation this webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. A titration experiment aims to determine the. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until. Titration Problems Equation.
From www.youtube.com
Calculations for Titration of Weak Base with Strong Acid, initial pH Titration Problems Equation a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. A titration experiment aims to determine the. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. titration is an analytical chemistry technique used to find an unknown concentration of an. Titration Problems Equation.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration Problems Equation a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. calculate the ph for the strong acid/strong base titration. Titration Problems Equation.
From chp090.chemistry.wustl.edu
Redox Titration Problem Titration Problems Equation Titration of a weak acid with a strong base. titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). this webpage describes a procedure called titration, which can be used to find the molarity of. Titration Problems Equation.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Problems Equation this webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. this video walks through common. Titration Problems Equation.
From www.youtube.com
Basic Titrations Chemistry Tutorial YouTube Titration Problems Equation 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration of a weak acid with a strong base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution. Titration Problems Equation.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2145706 Titration Problems Equation the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. Titration Problems Equation.
From www.youtube.com
Titration Calculations YouTube Titration Problems Equation A titration experiment aims to determine the. Titration of a weak acid with a strong base. this webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by. Titration Problems Equation.
From www.youtube.com
Acid Base titration problem solving YouTube Titration Problems Equation calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a. Titration Problems Equation.
From www.slideserve.com
PPT Steps for solving titration problems PowerPoint Presentation Titration Problems Equation this webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. calculate the ph for the strong acid/strong base titration between 50.0 ml of. Titration Problems Equation.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Problems Equation Titration of a weak acid with a strong base. this webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Titration Problems Equation.
From www.youtube.com
5 Solving Titration problems with acids and bases YouTube Titration Problems Equation Titration of a weak acid with a strong base. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: this video walks through common examples of calculation problems for titration. this webpage describes a procedure called titration,. Titration Problems Equation.
From www.chegg.com
Solved Here is a titration curve for 10.00 mL of an unknown Titration Problems Equation calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Titration of a weak acid with a strong base. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the. Titration Problems Equation.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Problems Equation this video walks through common examples of calculation problems for titration. titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). Titration of a weak acid with a strong base. calculate the ph for. Titration Problems Equation.
From chem.libretexts.org
Titration of A Strong Acid With A Strong Base Chemistry LibreTexts Titration Problems Equation this video walks through common examples of calculation problems for titration. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration of a weak acid with a strong base. titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand). Titration Problems Equation.
From dxompffry.blob.core.windows.net
Titration Formula Class 12 at Donald Rios blog Titration Problems Equation this webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. Titration of a weak acid with a strong base. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. the above equation works only for neutralizations in which there is a 1:1 ratio between the. Titration Problems Equation.
From www.youtube.com
AP Chemistry Titration Graph problem worksheet review YouTube Titration Problems Equation titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the. Titration Problems Equation.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Problems Equation A titration experiment aims to determine the. titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. Titration of a weak acid with a strong base. . Titration Problems Equation.
From mungfali.com
Acid Base Titration Formula Titration Problems Equation calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the.. Titration Problems Equation.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Problems Equation titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). this video walks through common examples of calculation problems for titration. this webpage describes a procedure called titration, which can be used to find. Titration Problems Equation.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration Problems Equation Titration of a weak acid with a strong base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. A titration experiment aims to determine the. calculate the ph for the. Titration Problems Equation.
From www.chegg.com
Solved To review how titration calculations work, here's a Titration Problems Equation a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. this webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid. Titration Problems Equation.
From www.youtube.com
acids and bases examples of titration problems YouTube Titration Problems Equation the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. Titration Problems Equation.
From mavink.com
Acid Base Titration Equation Titration Problems Equation this video walks through common examples of calculation problems for titration. Titration of a weak acid with a strong base. A titration experiment aims to determine the. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a. Titration Problems Equation.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration Problems Equation this video walks through common examples of calculation problems for titration. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. A titration experiment aims to determine the. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: . Titration Problems Equation.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Problems Equation A titration experiment aims to determine the. this video walks through common examples of calculation problems for titration. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. . Titration Problems Equation.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Problems Equation the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). a titration is a. Titration Problems Equation.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Problems Equation this webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. this video walks through common examples of calculation problems for titration. a titration is a volumetric technique in which a solution of one reactant. Titration Problems Equation.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID3459790 Titration Problems Equation this webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. A titration experiment aims to determine the. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 0.00 ml, 15.0 ml, 25.0 ml, and. Titration Problems Equation.
From www.youtube.com
Acidbase titration problem 1 YouTube Titration Problems Equation calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Titration of a weak acid with a strong base. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the. Titration Problems Equation.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration Problems Equation the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). a titration is a. Titration Problems Equation.