Gas Constant Volume . All of the empirical gas relationships are special. the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. the ideal gas law. volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume.
from www1.grc.nasa.gov
the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: All of the empirical gas relationships are special. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. the ideal gas law. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature.
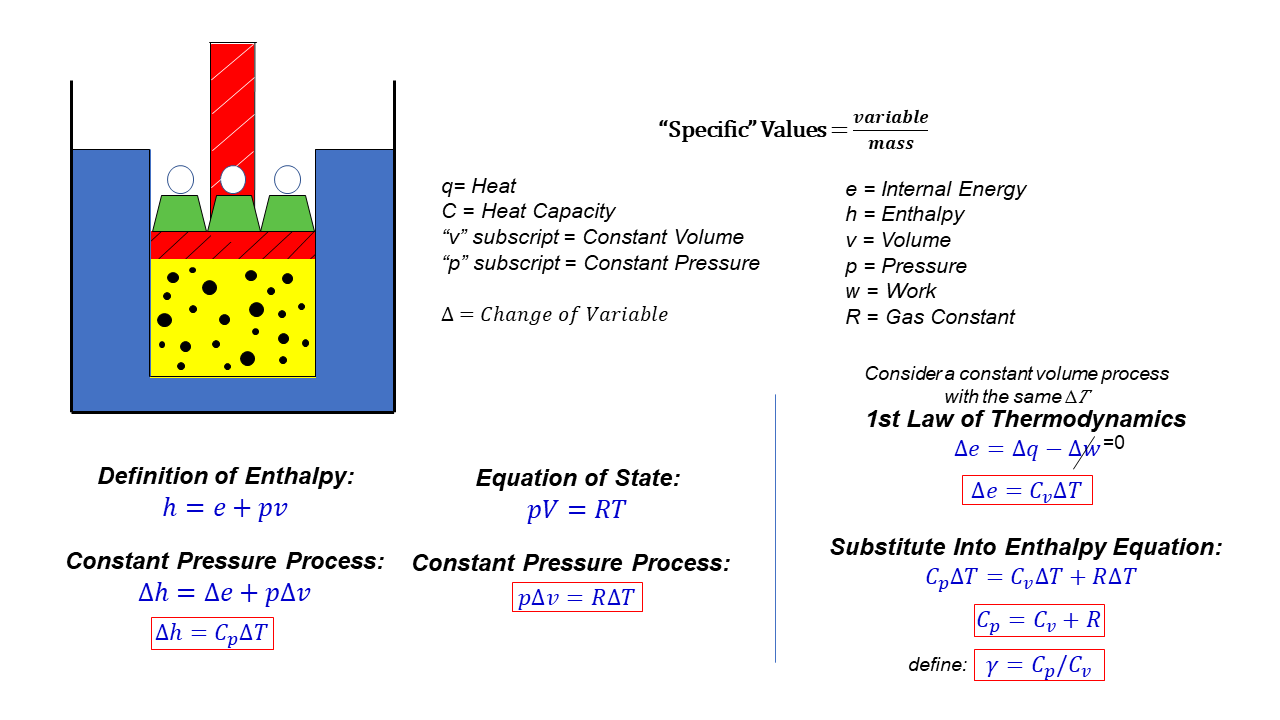
Specific Heats cp and cv Glenn Research Center NASA
Gas Constant Volume the ideal gas law. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. the ideal gas law. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: All of the empirical gas relationships are special. volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin.
From www.chegg.com
A Refrigerator Uses An Ideal Gas With Constant Vol... Gas Constant Volume the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. the ideal gas law. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. you can use the ideal gas volume calculator to find the molar volume of an ideal. Gas Constant Volume.
From www.nagwa.com
Question Video Calculating Gas Volume after It Is Heated at Constant Gas Constant Volume volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. . Gas Constant Volume.
From www.toppr.com
The initial pressure and volume of a given mass of an ideal gas (with Gas Constant Volume the ideal gas law. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: the volume of 1 mol of an ideal gas at stp is. Gas Constant Volume.
From upload.independent.com
How To Draw A Pv Diagram Gas Constant Volume The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. . Gas Constant Volume.
From ucscphysicsdemo.sites.ucsc.edu
Constant Volume Gas Thermometer UCSC Physics Demonstration Room Gas Constant Volume a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. All of the empirical gas relationships are special. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. the ideal gas equation relates the pressure and volume. Gas Constant Volume.
From chem.libretexts.org
7.3 The Gas Laws Chemistry LibreTexts Gas Constant Volume The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. . Gas Constant Volume.
From electrigjh.blogspot.com
Ideal Gas Law R Values Create A Function To Convert R (ideal Gas Gas Constant Volume you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in. Gas Constant Volume.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an Gas Constant Volume volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. the ideal gas law. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. All of the empirical gas relationships are special. you can use the ideal gas volume calculator. Gas Constant Volume.
From melonyy-upset.blogspot.com
Ideal Gas Law Constant R Values physical chemistry What volume does Gas Constant Volume you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and. Gas Constant Volume.
From www.markedbyteachers.com
Investigating the relationship between pressure, volume and temperature Gas Constant Volume All of the empirical gas relationships are special. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. The volume (v) occupied by n moles of any gas has a. Gas Constant Volume.
From nigerianscholars.com
Phase Changes Temperature, Theory, and Gas Laws Gas Constant Volume volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: . Gas Constant Volume.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Gas Constant Volume you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. . Gas Constant Volume.
From physics.stackexchange.com
thermodynamics Using the constantgas volume thermometer? Physics Gas Constant Volume The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. . Gas Constant Volume.
From quinnqusyki.blogspot.com
8.314 Gas Constant A Balloon Is Filled With 1 Mole Of Helium Gas At Gas Constant Volume volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: All. Gas Constant Volume.
From www.nagwa.com
Question Video Calculating the Change of the Volume of a Gas under Gas Constant Volume a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. the ideal gas law. you can use the ideal gas volume calculator to find the molar volume of. Gas Constant Volume.
From askfilo.com
An ideal gas is expanding such that PT2= constant. The coefficient of vol.. Gas Constant Volume a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar. Gas Constant Volume.
From www.studocu.com
Processes of Ideal Gas PROCESSES OF IDEAL GASES Constant Volume Gas Constant Volume All of the empirical gas relationships are special. the ideal gas law. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. a logical corollary to avogadro's hypothesis (sometimes called. Gas Constant Volume.
From www.chem.fsu.edu
Gas Laws Gas Constant Volume you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and. Gas Constant Volume.
From www.britannica.com
Ideal gas Definition, Equation, Properties, & Facts Britannica Gas Constant Volume The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard. Gas Constant Volume.
From exogbxcvg.blob.core.windows.net
Gas Constant Natural Gas at Elizabeth Ralston blog Gas Constant Volume you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: All of the empirical gas relationships are special. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s. Gas Constant Volume.
From www.youtube.com
Constant Pressure Heating of a gas YouTube Gas Constant Volume the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: the ideal gas law. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. The volume (v) occupied by n moles of any gas has a pressure (p) at. Gas Constant Volume.
From socratic.org
Which graph shows the relationship between the temperature and volume Gas Constant Volume volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount. Gas Constant Volume.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation ID6652783 Gas Constant Volume the ideal gas law. volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. All of the empirical gas relationships are special. a logical corollary to avogadro's hypothesis (sometimes called. Gas Constant Volume.
From www.youtube.com
Constant Volume Heating of a Gas YouTube Gas Constant Volume All of the empirical gas relationships are special. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: the ideal gas law. you can use the. Gas Constant Volume.
From owlcation.com
The Theories and Behavior of Gas Owlcation Gas Constant Volume the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: the ideal gas law. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. you can use the ideal gas volume calculator to find the molar volume of. Gas Constant Volume.
From cartoondealer.com
Boyle's Law Showing The Pressure And Volume Relationship Vector Gas Constant Volume The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. . Gas Constant Volume.
From mmerevise.co.uk
The Ideal Gas Equation MME Gas Constant Volume the ideal gas law. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. volume of a gas is directly proportional to the amount of gas. Gas Constant Volume.
From socratic.org
A sample of a gas has a volume of 2.0 liters at a pressure of 1.0 Gas Constant Volume volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. . Gas Constant Volume.
From tasubtitlex.weebly.com
Blog Archives tasubtitleX Gas Constant Volume you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. volume of a gas is directly proportional to the amount of gas at a constant temperature and. Gas Constant Volume.
From www.tec-science.com
Isothermal process in a closed system tecscience Gas Constant Volume the ideal gas law. the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. volume of a gas is directly proportional to the amount of gas. Gas Constant Volume.
From www.tec-science.com
Specific heat capacity of gases (at constant volume or pressure) tec Gas Constant Volume you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. the ideal gas law. All of the empirical gas relationships are special. The volume (v) occupied by n moles. Gas Constant Volume.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii Gas Constant Volume the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: the ideal gas law. All of the empirical gas relationships are special. the volume of 1 mol of an ideal gas at stp is 22.41 l, the standard molar volume. you can use the ideal gas. Gas Constant Volume.
From www.linstitute.net
IB DP Chemistry SL复习笔记1.2.3 Avogadro's Law & Molar Gas Volume翰林国际教育 Gas Constant Volume the ideal gas equation relates the pressure and volume of an ideal gas to the number of moles and temperature: All of the empirical gas relationships are special. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. the volume of 1 mol of an ideal gas. Gas Constant Volume.
From www1.grc.nasa.gov
Specific Heats cp and cv Glenn Research Center NASA Gas Constant Volume you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard temperature. the ideal gas law. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. The volume (v) occupied by n moles of any gas has a pressure. Gas Constant Volume.
From cartoondealer.com
Boyle's Law, Relationship Between Pressure And Volume Of Gas At Gas Constant Volume volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of. you can use the ideal gas volume calculator to find the molar volume of an ideal gas at standard. Gas Constant Volume.