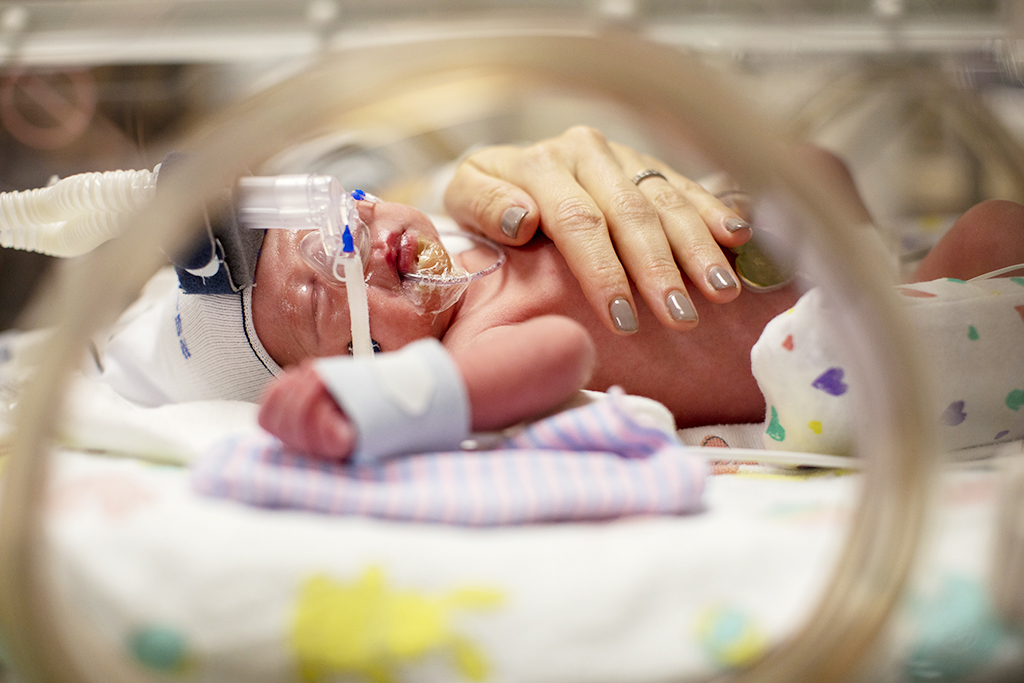
Premature Baby Pda Closure . 2 closure of the ductus occurs within hours in the majority. The device can be safely implanted to help seal the. Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. Procedural pda closure may be considered in extremely preterm infants (<28 weeks gestational age) requiring invasive. Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g; The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. As the fetal lungs do not participate in. Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature. A system of shunts in the growing fetus ensures optimal oxygen delivery to vital organs. Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality. In preterm infants, da closure frequently fails to occur because of immature structures and responses to constrictive.
from www.chla.org
Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature. Procedural pda closure may be considered in extremely preterm infants (<28 weeks gestational age) requiring invasive. Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. As the fetal lungs do not participate in. A system of shunts in the growing fetus ensures optimal oxygen delivery to vital organs. In preterm infants, da closure frequently fails to occur because of immature structures and responses to constrictive. The device can be safely implanted to help seal the. Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g; Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality.
Does Piccolo PDA Closure Improve for Babies? Children’s
Premature Baby Pda Closure Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g; 2 closure of the ductus occurs within hours in the majority. The device can be safely implanted to help seal the. Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality. Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature. In preterm infants, da closure frequently fails to occur because of immature structures and responses to constrictive. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. A system of shunts in the growing fetus ensures optimal oxygen delivery to vital organs. As the fetal lungs do not participate in. Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g; Procedural pda closure may be considered in extremely preterm infants (<28 weeks gestational age) requiring invasive.
From www.youtube.com
Device closure of PDA YouTube Premature Baby Pda Closure Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g; The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality. A system of shunts in the growing fetus ensures optimal oxygen delivery. Premature Baby Pda Closure.
From www.youtube.com
A Suffering Journey of PDA Closure in a 1300g Premature Baby YouTube Premature Baby Pda Closure The device can be safely implanted to help seal the. Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature. Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality. Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g; 2 closure of. Premature Baby Pda Closure.
From www.thelondoneconomic.com
One of UK’s most premature babies defied odds after being born at 22 Premature Baby Pda Closure 2 closure of the ductus occurs within hours in the majority. As the fetal lungs do not participate in. The device can be safely implanted to help seal the. Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. A system of shunts. Premature Baby Pda Closure.
From www.jscai.org
A Global Perspective on PDA Management in the Extremely Premature Premature Baby Pda Closure Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality. As the fetal lungs do not participate in. Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. 2 closure of the ductus occurs within hours in the majority. Recently, fda and. Premature Baby Pda Closure.
From www.structuralheart.abbott
Patent Ductus Arteriosus Closure in Premature Infants Premature Baby Pda Closure Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda. Premature Baby Pda Closure.
From www.researchgate.net
Case demonstration in a premature baby with a gestational age of 26 ± Premature Baby Pda Closure In preterm infants, da closure frequently fails to occur because of immature structures and responses to constrictive. The device can be safely implanted to help seal the. As the fetal lungs do not participate in. Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality. Transcatheter pda closure is safe and efficacious in premature infants using the ka micro. Premature Baby Pda Closure.
From www.nytimes.com
Better Care for Premature Babies Also Means Harder Choices The New Premature Baby Pda Closure A system of shunts in the growing fetus ensures optimal oxygen delivery to vital organs. Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g;. Premature Baby Pda Closure.
From answers.childrenshospital.org
PDA transcatheter closure in preemies Boston Children's Answers Premature Baby Pda Closure Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. 2 closure of the ductus occurs within hours in the majority. The device can be safely implanted to help seal the. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device. Premature Baby Pda Closure.
From www.hilarispublisher.com
vasculitisclosure Premature Baby Pda Closure 2 closure of the ductus occurs within hours in the majority. Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality. Procedural pda closure may be considered in extremely preterm infants (<28 weeks gestational age) requiring invasive. Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature. The device can be. Premature Baby Pda Closure.
From www.vrogue.co
Further Experience With Catheter Closure Of Patent Du vrogue.co Premature Baby Pda Closure 2 closure of the ductus occurs within hours in the majority. Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. In preterm infants, da closure frequently fails to occur because of immature structures and responses to constrictive. The device can be safely. Premature Baby Pda Closure.
From www.youtube.com
Surprise Recovery of Premature Baby After PDA Ligation YouTube Premature Baby Pda Closure Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature. The device can be safely implanted to help seal the. As the fetal lungs do not participate in. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Transcatheter pda closure is safe. Premature Baby Pda Closure.
From www.healthclips.com
Text Patent Ductus Arteriosus (PDA) in Premature Infants Premature Baby Pda Closure Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. A system of shunts in the growing fetus ensures optimal oxygen delivery to vital organs. Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g;. Premature Baby Pda Closure.
From www.childrenscolorado.org
Patent Ductus Arteriosus (PDA) Children's Hospital Colorado Premature Baby Pda Closure Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g; Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality. Recently, fda and ce. Premature Baby Pda Closure.
From www.vrogue.co
Patent Ductus Arteriosis Pda Series Procedure Medline vrogue.co Premature Baby Pda Closure The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. 2 closure of the ductus occurs within hours in the majority. In preterm infants, da closure frequently fails to occur because of immature structures and responses to constrictive. Procedural pda closure may be considered in extremely preterm infants (<28 weeks gestational. Premature Baby Pda Closure.
From www.frontiersin.org
Frontiers Anterior Minithoracotomy vs. Transcatheter Closure of Premature Baby Pda Closure As the fetal lungs do not participate in. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. A system of shunts in the growing fetus ensures optimal oxygen delivery to vital organs. Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g;. Premature Baby Pda Closure.
From milaap.org
Crowdfunding story collage 75 ksyaxm 1676917382 Premature Baby Pda Closure Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g; The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature. Patent ductus arteriosus (pda) is. Premature Baby Pda Closure.
From www.chla.org
Does Piccolo PDA Closure Improve for Babies? Children’s Premature Baby Pda Closure Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature. The device can be safely implanted to help seal the. Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g; The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is. Premature Baby Pda Closure.
From discover.hubpages.com
Patent Ductus Arteriosus Pediatric Heart Defect HubPages Premature Baby Pda Closure In preterm infants, da closure frequently fails to occur because of immature structures and responses to constrictive. 2 closure of the ductus occurs within hours in the majority. Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature. Reported successful pda closure via transcatheter route in 20 out of 23 premature infants. Premature Baby Pda Closure.
From wecareindia.com
Pediatric PDA Closure Device Surgery,Price PDA Closure Device Premature Baby Pda Closure In preterm infants, da closure frequently fails to occur because of immature structures and responses to constrictive. The device can be safely implanted to help seal the. Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature. Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean. Premature Baby Pda Closure.
From childrenheartcare.com
Best PDA Surgery in Delhi Dr. Gaurav Agrawal Premature Baby Pda Closure 2 closure of the ductus occurs within hours in the majority. Procedural pda closure may be considered in extremely preterm infants (<28 weeks gestational age) requiring invasive. A system of shunts in the growing fetus ensures optimal oxygen delivery to vital organs. Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature.. Premature Baby Pda Closure.
From www.jpeds.com
Percutaneous Closure of the Patent Ductus Arteriosus in Very Low Weight Premature Baby Pda Closure The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. The device can be safely implanted to help seal the. A system of shunts in the growing fetus ensures optimal oxygen delivery to vital organs. As the fetal lungs do not participate in. Transcatheter pda closure is safe and efficacious in. Premature Baby Pda Closure.
From www.youtube.com
PDA Cath Procedure Repairs Premature Baby's Heart Juniper's Story at Premature Baby Pda Closure Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality. As the fetal lungs do not participate in. Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure. Premature Baby Pda Closure.
From drraghu.com
Patent Ductus Arteriosus (PDA) Symptoms and Risk Factors Dr Raghu Premature Baby Pda Closure Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature. The device can be safely implanted to help seal the. Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g; Procedural pda closure may be considered in extremely preterm infants (<28 weeks gestational age). Premature Baby Pda Closure.
From childrenheartcare.com
PDA Management Dr. Gaurav Agrawal Premature Baby Pda Closure 2 closure of the ductus occurs within hours in the majority. Procedural pda closure may be considered in extremely preterm infants (<28 weeks gestational age) requiring invasive. Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality. As the fetal lungs do not participate in. The device can be safely implanted to help seal the. Reported successful pda closure. Premature Baby Pda Closure.
From www.ahajournals.org
Premature Closure of the Foramen Ovale and Ductus Arteriosus in a Fetus Premature Baby Pda Closure 2 closure of the ductus occurs within hours in the majority. The device can be safely implanted to help seal the. Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g; Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature. Procedural pda closure. Premature Baby Pda Closure.
From www.structuralheart.abbott
PDA Closure in infants Premature Baby Pda Closure Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g; 2 closure of the ductus occurs within hours in the majority. Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. As the fetal lungs. Premature Baby Pda Closure.
From ctsurgerypatients.org
Patent Ductus Arteriosus The Patient Guide to Heart, Lung, and Premature Baby Pda Closure Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. In preterm infants,. Premature Baby Pda Closure.
From clinicalconnection.hopkinsmedicine.org
Patent Ductus Arteriosus in Preterm Infants Johns Hopkins Medicine Premature Baby Pda Closure A system of shunts in the growing fetus ensures optimal oxygen delivery to vital organs. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g; In preterm infants, da closure frequently fails to. Premature Baby Pda Closure.
From www.semanticscholar.org
Figure 1 from Transcatheter closure of PDA in premature babies less Premature Baby Pda Closure The device can be safely implanted to help seal the. Recently, fda and ce approved the amplatzer piccolo occluder® device for the transcatheter closure of pda in premature. In preterm infants, da closure frequently fails to occur because of immature structures and responses to constrictive. As the fetal lungs do not participate in. Procedural pda closure may be considered in. Premature Baby Pda Closure.
From www.heart.org
Patent Ductus Arteriosus (PDA) American Heart Association Premature Baby Pda Closure 2 closure of the ductus occurs within hours in the majority. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality. Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp. Premature Baby Pda Closure.
From www.semanticscholar.org
Figure 2 from Transcatheter closure of PDA in premature babies less Premature Baby Pda Closure Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. As the fetal lungs do not participate in. Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality. Procedural pda closure may be considered in extremely preterm infants (<28 weeks gestational age). Premature Baby Pda Closure.
From www.researchgate.net
Measurement of patent ductus arteriosus (PDA) size on suprasternal Premature Baby Pda Closure In preterm infants, da closure frequently fails to occur because of immature structures and responses to constrictive. Patent ductus arteriosus (pda) is associated with morbidity 1 and mortality. Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. Reported successful pda closure via. Premature Baby Pda Closure.
From www.chop.edu
Patent Ductus Arteriosus (PDA) Children's Hospital of Philadelphia Premature Baby Pda Closure Procedural pda closure may be considered in extremely preterm infants (<28 weeks gestational age) requiring invasive. Reported successful pda closure via transcatheter route in 20 out of 23 premature infants (mean weight 1,250 g; The device can be safely implanted to help seal the. As the fetal lungs do not participate in. A system of shunts in the growing fetus. Premature Baby Pda Closure.
From www.researchgate.net
(PDF) Transcatheter Closure of Patent Ductus Arteriosus in Under 6 kg Premature Baby Pda Closure A system of shunts in the growing fetus ensures optimal oxygen delivery to vital organs. In preterm infants, da closure frequently fails to occur because of immature structures and responses to constrictive. Transcatheter pda closure is safe and efficacious in premature infants using the ka micro plug, piccolo device, and mvp with exceptionally low mortality and acute to medium. Recently,. Premature Baby Pda Closure.
From www.heart.org
Patent Ductus Arteriosus (PDA) American Heart Association Premature Baby Pda Closure The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Procedural pda closure may be considered in extremely preterm infants (<28 weeks gestational age) requiring invasive. A system of shunts in the growing fetus ensures optimal oxygen delivery to vital organs. 2 closure of the ductus occurs within hours in the. Premature Baby Pda Closure.