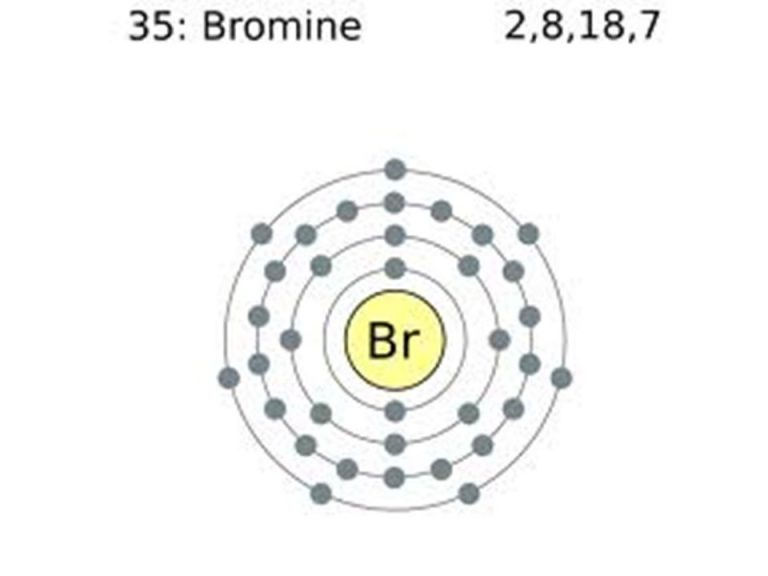
Bromine Electron Distribution . The bromine electron configuration, denoted as 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within the atom. Br (bromine) is an element with position number 35 in the periodic table. It was the first element to be extracted. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. Also, we will provide the pictures of the same. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. Located in the iv period.
from periodictable.me
It was the first element to be extracted. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The bromine electron configuration, denoted as 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within the atom. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. Also, we will provide the pictures of the same. Br (bromine) is an element with position number 35 in the periodic table. Located in the iv period. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2.
Bromine Electron Configuration (Br) with Orbital Diagram
Bromine Electron Distribution The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. Br (bromine) is an element with position number 35 in the periodic table. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Located in the iv period. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. It was the first element to be extracted. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. The bromine electron configuration, denoted as 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within the atom. Also, we will provide the pictures of the same.
From www.shutterstock.com
Bohr Model Bromine Atom Electron Structure เวกเตอร์สต็อก (ปลอดค่า Bromine Electron Distribution Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. It was the first element to be extracted. Located in the iv period. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Bromine atoms have 35 electrons and the. Bromine Electron Distribution.
From www.alamy.com
3d render of atom structure of bromine isolated over white background Bromine Electron Distribution Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Br (bromine) is an element with position number 35 in the periodic table. Also, we will provide the pictures of the same. Since bromine has 7 valence. Bromine Electron Distribution.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Electron Distribution Located in the iv period. Also, we will provide the pictures of the same. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. In this article today. Bromine Electron Distribution.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Distribution Also, we will provide the pictures of the same. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. It was the first element to be extracted. In this article today we are going to tell you about the. Bromine Electron Distribution.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Distribution Located in the iv period. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Br (bromine) is an element with position number 35 in the periodic table. It was the first element to be extracted. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. The bromine electron configuration, denoted as 4s 2. Bromine Electron Distribution.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Electron Distribution It was the first element to be extracted. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. Located in the iv period. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Also, we will provide the pictures of. Bromine Electron Distribution.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Distribution In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Br (bromine) is an element with position number 35 in the periodic table. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. The bromine electron configuration, denoted as 4s 2 3d 10. Bromine Electron Distribution.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Bromine Electron Distribution Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. It was the first element to be extracted. Also, we will provide the pictures of the same. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The bromine electron configuration, denoted as. Bromine Electron Distribution.
From mavink.com
Lewis Dot Diagram For Bromine Bromine Electron Distribution In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. It was the first element to be extracted. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii. Bromine Electron Distribution.
From www.dreamstime.com
Bromine Atom, with Mass and Energy Levels. Stock Vector Illustration Bromine Electron Distribution Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The bromine electron configuration, denoted as 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within the atom. Also, we will provide the pictures of the same. In this. Bromine Electron Distribution.
From lambdageeks.com
Bromine Electron Configuration 7 Easy Steps on How to Write Bromine Electron Distribution It was the first element to be extracted. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Br (bromine) is an element with position number 35 in the periodic table. The bromine electron configuration, denoted as 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6. Bromine Electron Distribution.
From www.americanelements.com
Bromine (Br) AMERICAN ELEMENTS Bromine Electron Distribution Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. Also, we will provide the pictures of the same.. Bromine Electron Distribution.
From autoctrls.com
Understanding the Bromine Electron Dot Diagram A Comprehensive Guide Bromine Electron Distribution The bromine electron configuration, denoted as 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within the atom. Br (bromine) is an element with position number 35 in the periodic table. In this article today we are going to tell. Bromine Electron Distribution.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Electron Distribution Also, we will provide the pictures of the same. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. Br (bromine) is an element with position number 35 in the periodic table. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Bromine. Bromine Electron Distribution.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Distribution Also, we will provide the pictures of the same. It was the first element to be extracted. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Located in the iv period. Bromine atoms have 35. Bromine Electron Distribution.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Electron Distribution Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. The bromine electron configuration, denoted as 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within the atom. Also, we will provide the pictures of. Bromine Electron Distribution.
From www.shutterstock.com
Symbol Electron Diagram Bromine Illustration Stock Vector (Royalty Free Bromine Electron Distribution Br (bromine) is an element with position number 35 in the periodic table. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. The bromine electron configuration, denoted as 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise. Bromine Electron Distribution.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy Bromine Electron Distribution Also, we will provide the pictures of the same. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. It was the first element to be extracted. The bromine electron configuration, denoted. Bromine Electron Distribution.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Electron Distribution Located in the iv period. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table.. Bromine Electron Distribution.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Electron Distribution The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. Br (bromine) is an element with position number 35 in the periodic table. In this article today we are going to tell you about the electron. Bromine Electron Distribution.
From www.youtube.com
Electron Configuration of Bromine Br Lesson YouTube Bromine Electron Distribution The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. The bromine electron configuration, denoted as 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within the atom. Br (bromine) is an element with. Bromine Electron Distribution.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Electron Distribution Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. Located in the iv period. Since bromine has 7. Bromine Electron Distribution.
From app.emaze.com
Bromine ) on emaze Bromine Electron Distribution The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. It was the first element to be extracted. The bromine electron configuration, denoted as 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Bromine Electron Distribution.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Bromine Electron Distribution Also, we will provide the pictures of the same. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. Br (bromine) is an element with position number 35 in the periodic table. It was the first element to be extracted. The ground state electron configuration of ground state gaseous neutral bromine is. Bromine Electron Distribution.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine Electron Distribution Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. It was the first element to be extracted. Br (bromine) is an element with position number 35 in the periodic table. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. Located in the iv period. In. Bromine Electron Distribution.
From iperiodictable.com
How Can We Find A Electron Configuration For Bromine (Br) Bromine Electron Distribution Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. It was the first element to be extracted. Also, we will provide the pictures of the same. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Bromine atoms have 35 electrons and the shell structure. Bromine Electron Distribution.
From www.dreamstime.com
Element of Bromine stock vector. Illustration of college 104400483 Bromine Electron Distribution In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Located in the iv period. Br (bromine) is an. Bromine Electron Distribution.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Distribution Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. The bromine electron configuration, denoted as 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within the atom. Located in the iv period. In this. Bromine Electron Distribution.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Distribution Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. Also, we will provide the pictures of the same. In this article today we are going to tell you about the electron configuration of bromine, its orbital. Bromine Electron Distribution.
From www.slideserve.com
PPT Draw Bromine Electron Shell Configuration 1s 2 2s 2 2p 6 3s 2 3p Bromine Electron Distribution The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. It was the first element to be extracted. Br (bromine) is an element with position number 35 in the periodic table. Also, we will provide the pictures of the same. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with. Bromine Electron Distribution.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Electron Distribution Also, we will provide the pictures of the same. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. It was the first element to be extracted. Located in the iv period. Br (bromine) is an element with position number 35 in the periodic table. The ground state electron configuration of ground. Bromine Electron Distribution.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Bromine Electron Distribution In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Located in the iv period. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. The ground state electron. Bromine Electron Distribution.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Distribution Br (bromine) is an element with position number 35 in the periodic table. It was the first element to be extracted. Also, we will provide the pictures of the same. The bromine electron configuration, denoted as 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases. Bromine Electron Distribution.
From www.alamy.com
Chemist atom of Bromine diagram Stock Vector Image & Art Alamy Bromine Electron Distribution It was the first element to be extracted. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Br (bromine) is an element with position number 35 in the periodic table. Since bromine has 7 valence electrons,. Bromine Electron Distribution.
From www.youtube.com
Super Trick Electronic configuration of Bromine Bromine electronic Bromine Electron Distribution The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Br (bromine) is an element with position number 35 in the periodic table. Since bromine has 7 valence electrons, the 4s orbital will be completely filled with 2. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the. Bromine Electron Distribution.