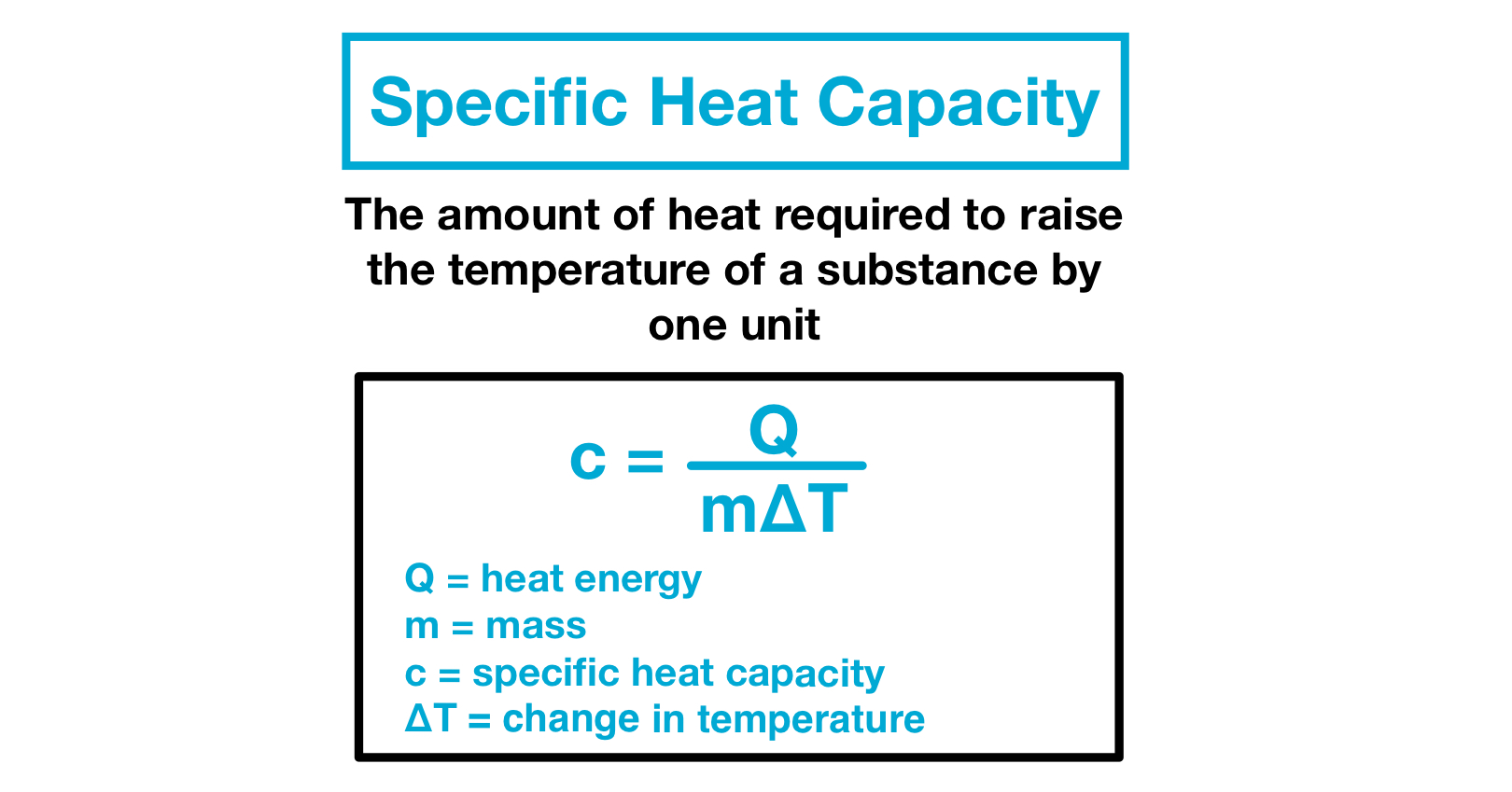
What Is A Low Heat Capacity . A substance with a small heat. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. Heat capacity, ratio of heat absorbed by a material to the temperature change. It plays a crucial role in understanding. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. It is usually expressed as calories per degree in. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. In equation form, heat capacity c is c = m c,. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance to.
from www.expii.com
Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance to. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It is usually expressed as calories per degree in. In equation form, heat capacity c is c = m c,. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. A substance with a small heat. It plays a crucial role in understanding. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. Heat capacity, ratio of heat absorbed by a material to the temperature change.
Heat Capacity of Water — Overview & Importance Expii
What Is A Low Heat Capacity The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance to. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance to. It plays a crucial role in understanding. In equation form, heat capacity c is c = m c,. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. It is usually expressed as calories per degree in. A substance with a small heat. Heat capacity, ratio of heat absorbed by a material to the temperature change.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is A Low Heat Capacity In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. It plays a crucial role in understanding. In equation form, heat capacity c is c = m c,. The heat capacity of a. What Is A Low Heat Capacity.
From pt.slideshare.net
Heat Capacity What Is A Low Heat Capacity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It plays a crucial role in understanding. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change. What Is A Low Heat Capacity.
From www.tes.com
AQA Specific Heat Capacity theory New GCSE Teaching Resources What Is A Low Heat Capacity A substance with a small heat. It plays a crucial role in understanding. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one. What Is A Low Heat Capacity.
From www.slideserve.com
PPT THERMOCHEMISTRY Thermodynamics The study of Heat and Work and What Is A Low Heat Capacity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It plays a crucial role in understanding. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. Heat capacity is the amount of heat necessary to. What Is A Low Heat Capacity.
From mavink.com
Specific Heat Capacity Diagram What Is A Low Heat Capacity Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. The heat capacity of a substance describes how its temperature changes as. What Is A Low Heat Capacity.
From www.slideserve.com
PPT Properties of Materials PowerPoint Presentation, free download What Is A Low Heat Capacity In equation form, heat capacity c is c = m c,. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. A substance with a small heat. Heat capacity, ratio of heat absorbed by a material to the temperature change. It. What Is A Low Heat Capacity.
From surfaceareaworksheet.blogspot.com
Specific Heat And Heat Capacity Worksheet Answers What Is A Low Heat Capacity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. In words, heat capacity is the substance's ability to resist change. What Is A Low Heat Capacity.
From www.downwindkennels.com
Specific Heat Capacity What Is A Low Heat Capacity In equation form, heat capacity c is c = m c,. It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance to. A substance with a small heat. Heat capacity (usually denoted by a capital c, often with subscripts),. What Is A Low Heat Capacity.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources What Is A Low Heat Capacity In equation form, heat capacity c is c = m c,. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. A substance with a small heat. The heat capacity (c) of a body of matter is the quantity. What Is A Low Heat Capacity.
From studymind.co.uk
Heat Capacity Study Mind What Is A Low Heat Capacity Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or. What Is A Low Heat Capacity.
From www.slideserve.com
PPT Chapter 13 Global Weather Dynamics PowerPoint Presentation, free What Is A Low Heat Capacity In equation form, heat capacity c is c = m c,. A substance with a small heat. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to. What Is A Low Heat Capacity.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems What Is A Low Heat Capacity It plays a crucial role in understanding. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance to. The heat capacity (c) of a body of matter is the quantity of heat (q). What Is A Low Heat Capacity.
From www.youtube.com
Lecture 14 What is Heat Capacity? YouTube What Is A Low Heat Capacity The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount. What Is A Low Heat Capacity.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity What Is A Low Heat Capacity Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount. What Is A Low Heat Capacity.
From www.slideserve.com
PPT Chapter 5 Phonons II Thermal Properties PowerPoint What Is A Low Heat Capacity Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. In equation form, heat capacity c is c = m c,. It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it. What Is A Low Heat Capacity.
From www.slideserve.com
PPT Section 7.2—Calorimetry & Heat Capacity PowerPoint Presentation What Is A Low Heat Capacity A substance with a small heat. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance to. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Specific heat is defined as. What Is A Low Heat Capacity.
From www.slideserve.com
PPT Heat capacity PowerPoint Presentation, free download ID1419022 What Is A Low Heat Capacity Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. It plays a crucial role in understanding. It is usually expressed as calories per degree in. In equation form, heat capacity c is c = m c,. The heat capacity (c) of a body of matter is the quantity of heat (q). What Is A Low Heat Capacity.
From www.slideshare.net
Thermodynamics Heat and Temperature What Is A Low Heat Capacity In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. The heat capacity of. What Is A Low Heat Capacity.
From www.slideserve.com
PPT Lecture 5 PowerPoint Presentation, free download ID442102 What Is A Low Heat Capacity Heat capacity, ratio of heat absorbed by a material to the temperature change. In equation form, heat capacity c is c = m c,. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat,. What Is A Low Heat Capacity.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID4499939 What Is A Low Heat Capacity Heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories per degree in. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when. What Is A Low Heat Capacity.
From www.slideserve.com
PPT Heat capacity of the lattice PowerPoint Presentation, free What Is A Low Heat Capacity Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. A substance with a small heat. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. In words, heat capacity is the substance's. What Is A Low Heat Capacity.
From serc.carleton.edu
Heat Capacity What Is A Low Heat Capacity It is usually expressed as calories per degree in. A substance with a small heat. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Specific heat is. What Is A Low Heat Capacity.
From studylib.net
1.3 Specific Heat Capacity What Is A Low Heat Capacity The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance to. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. The heat capacity (c) of a. What Is A Low Heat Capacity.
From www.slideserve.com
PPT Thermal Properties PowerPoint Presentation, free download ID What Is A Low Heat Capacity Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. It is usually expressed as calories per degree in. A substance with a small heat. The heat capacity of a substance describes how its temperature changes as it absorbs or releases. What Is A Low Heat Capacity.
From www.slideserve.com
PPT Chapter 17 Thermochemistry PowerPoint Presentation, free What Is A Low Heat Capacity Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. It plays a crucial role in understanding. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the. What Is A Low Heat Capacity.
From www.researchgate.net
Temperature dependence of the heat capacity of alloy A1. (a What Is A Low Heat Capacity The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance to. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. A substance with a small heat. It is usually expressed as calories per degree in. Specific. What Is A Low Heat Capacity.
From www.slideserve.com
PPT Heat capacity and Specific Heat PowerPoint Presentation, free What Is A Low Heat Capacity Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance to. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences. What Is A Low Heat Capacity.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii What Is A Low Heat Capacity A substance with a small heat. It plays a crucial role in understanding. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. It is usually expressed as calories per degree in. Heat capacity is the amount of heat necessary to change the temperature of a. What Is A Low Heat Capacity.
From www.researchgate.net
Behavior of heat capacity curves in the low temperature region What Is A Low Heat Capacity In equation form, heat capacity c is c = m c,. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. Heat capacity (usually denoted by a. What Is A Low Heat Capacity.
From askfilo.com
The differences between specific heat capacity and heat capacity are.. What Is A Low Heat Capacity In equation form, heat capacity c is c = m c,. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. It is usually expressed as calories per degree in. The heat capacity (c) of a body of matter is the. What Is A Low Heat Capacity.
From www.differencebetween.com
Difference Between Heat Capacity and Specific Heat Compare the What Is A Low Heat Capacity It plays a crucial role in understanding. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity (c) of a body of matter. What Is A Low Heat Capacity.
From www.slideserve.com
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free What Is A Low Heat Capacity In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. It plays a crucial role in understanding. In equation form, heat capacity c is c = m c,. It is usually expressed as calories per degree in. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity,. What Is A Low Heat Capacity.
From www.tec-science.com
Specific heat capacity of selected substances tecscience What Is A Low Heat Capacity In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. Heat capacity, ratio of heat absorbed by a material to the temperature change. In equation form, heat capacity c is c = m. What Is A Low Heat Capacity.
From www.slideserve.com
PPT Heat capacity of the lattice PowerPoint Presentation, free What Is A Low Heat Capacity It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance to. A substance with a small heat. Specific heat is defined as the amount. What Is A Low Heat Capacity.
From www.slideshare.net
Heat Capacity What Is A Low Heat Capacity Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity, ratio of heat absorbed by a material to the temperature change. In equation form, heat capacity c is c = m c,. The heat capacity (c) of a body of matter is the. What Is A Low Heat Capacity.