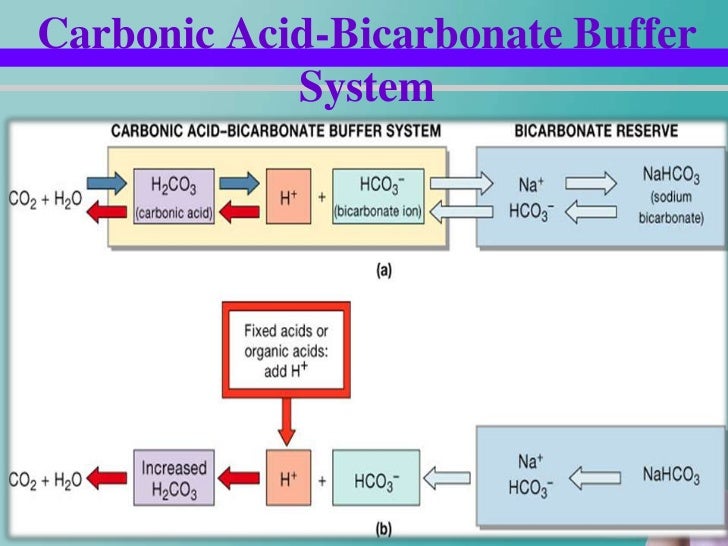
What Is Acid-Base Buffer System . This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. One mechanism the body uses to control blood ph involves the release of carbon dioxide from the. Define buffers and know the composition of different buffer systems. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Identify conjugate acid base pair. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations in the. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.
from www.slideshare.net
A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Define buffers and know the composition of different buffer systems. A buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations in the. One mechanism the body uses to control blood ph involves the release of carbon dioxide from the. Identify conjugate acid base pair. It is able to neutralize small amounts of added acid or base, thus. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial.
Buffer system
What Is Acid-Base Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations in the. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Identify conjugate acid base pair. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Define buffers and know the composition of different buffer systems. One mechanism the body uses to control blood ph involves the release of carbon dioxide from the. It is able to neutralize small amounts of added acid or base, thus.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Is Acid-Base Buffer System It is able to neutralize small amounts of added acid or base, thus. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Identify conjugate acid base pair. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. One mechanism the body uses. What Is Acid-Base Buffer System.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance What Is Acid-Base Buffer System A buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations in the. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Define buffers and know the composition of different buffer. What Is Acid-Base Buffer System.
From beta.learner.org
The Buffer System in the Blood (animation) Annenberg Learner What Is Acid-Base Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. One mechanism the body uses to control blood ph involves the release of carbon dioxide from the. Identify conjugate acid base pair. Define buffers and know the composition of different buffer systems. It is able to neutralize small. What Is Acid-Base Buffer System.
From labpedia.net
Acidbase Balance Part 2 Introduction of AcidBase Balance What Is Acid-Base Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in. What Is Acid-Base Buffer System.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Is Acid-Base Buffer System This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations in the. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A variety. What Is Acid-Base Buffer System.
From www.anaesthesiajournal.co.uk
Renal physiology acidbase balance Anaesthesia & Intensive Care Medicine What Is Acid-Base Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. It is able to neutralize small amounts of added acid or base, thus. A mixture of a weak acid and its conjugate base (or a mixture. What Is Acid-Base Buffer System.
From www.pinterest.com
Understanding Buffer Solution Chemistry A Complete Guide What Is Acid-Base Buffer System Define buffers and know the composition of different buffer systems. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. One mechanism the body uses to control blood ph involves the release of carbon dioxide from the. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities. What Is Acid-Base Buffer System.
From www.paediatricsandchildhealthjournal.co.uk
Understanding blood gases/acidbase balance Paediatrics and Child Health What Is Acid-Base Buffer System Define buffers and know the composition of different buffer systems. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It is able to neutralize small amounts of added acid or base, thus. Identify conjugate acid base pair. This characteristic makes buffers important. What Is Acid-Base Buffer System.
From www.youtube.com
1 Regulation of Blood pH By Buffer SystemsAcid Base Balance What Is Acid-Base Buffer System This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. A variety of buffering systems permits blood and other bodily fluids to maintain. What Is Acid-Base Buffer System.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Is Acid-Base Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Identify conjugate acid base pair. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion. What Is Acid-Base Buffer System.
From www.slideshare.net
Buffer system What Is Acid-Base Buffer System A buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations in the. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Define buffers and know the composition of different buffer. What Is Acid-Base Buffer System.
From ditki.com
Physiology Glossary Acids & Bases ditki medical & biological sciences What Is Acid-Base Buffer System This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic. What Is Acid-Base Buffer System.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 What Is Acid-Base Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations in the. A buffer is a solution that can resist ph change upon the addition of. What Is Acid-Base Buffer System.
From www.slideshare.net
Acid base balance What Is Acid-Base Buffer System It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer is a solution that maintains the stability of a system’s ph. What Is Acid-Base Buffer System.
From www.studypool.com
SOLUTION Acid base balance buffer system Studypool What Is Acid-Base Buffer System It is able to neutralize small amounts of added acid or base, thus. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. One mechanism the body uses to control blood ph involves the release of carbon dioxide from the. A buffer is. What Is Acid-Base Buffer System.
From lifeboat.com
Acids, Bases, PH, Buffers What Is Acid-Base Buffer System Define buffers and know the composition of different buffer systems. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It is able to neutralize small amounts of. What Is Acid-Base Buffer System.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID1958325 What Is Acid-Base Buffer System This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called. What Is Acid-Base Buffer System.
From www.youtube.com
Acid Base Physiology Part One Basics Buffers Renal Physiology What Is Acid-Base Buffer System One mechanism the body uses to control blood ph involves the release of carbon dioxide from the. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations in the.. What Is Acid-Base Buffer System.
From courses.lumenlearning.com
Buffers Chemistry What Is Acid-Base Buffer System It is able to neutralize small amounts of added acid or base, thus. One mechanism the body uses to control blood ph involves the release of carbon dioxide from the. Define buffers and know the composition of different buffer systems. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate. What Is Acid-Base Buffer System.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online What Is Acid-Base Buffer System A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. One mechanism the body uses to control blood ph involves the release of carbon dioxide from the. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Identify conjugate. What Is Acid-Base Buffer System.
From www.youtube.com
26 Acid Base Buffers YouTube What Is Acid-Base Buffer System A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations in the. A mixture of a weak acid and its conjugate base (or a mixture of. What Is Acid-Base Buffer System.
From saylordotorg.github.io
Buffers What Is Acid-Base Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. Define buffers and know the composition of different buffer systems. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer is a solution that can resist ph change upon the addition. What Is Acid-Base Buffer System.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation ID496487 What Is Acid-Base Buffer System One mechanism the body uses to control blood ph involves the release of carbon dioxide from the. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A buffer. What Is Acid-Base Buffer System.
From philschatz.com
AcidBase Balance · Anatomy and Physiology What Is Acid-Base Buffer System One mechanism the body uses to control blood ph involves the release of carbon dioxide from the. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. Identify conjugate acid base pair. A. What Is Acid-Base Buffer System.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium What Is Acid-Base Buffer System Define buffers and know the composition of different buffer systems. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added. What Is Acid-Base Buffer System.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks What Is Acid-Base Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a chemical system that prevents a radical change in fluid ph by dampening. What Is Acid-Base Buffer System.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free What Is Acid-Base Buffer System This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It is able to neutralize small amounts of added acid or base, thus. A variety of buffering systems. What Is Acid-Base Buffer System.
From deporecipe.co
10x Protein Running Buffer Recipe Deporecipe.co What Is Acid-Base Buffer System This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution. What Is Acid-Base Buffer System.
From www.slideshare.net
Buffer system What Is Acid-Base Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations in the. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer. What Is Acid-Base Buffer System.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 What Is Acid-Base Buffer System It is able to neutralize small amounts of added acid or base, thus. Define buffers and know the composition of different buffer systems. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an. What Is Acid-Base Buffer System.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Is Acid-Base Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Define buffers and know the composition of different buffer systems. Identify conjugate acid base pair. One mechanism the body uses to control blood. What Is Acid-Base Buffer System.
From labpedia.net
Acidbase Balance Part 1 A Introduction to the AcidBase Balance What Is Acid-Base Buffer System Identify conjugate acid base pair. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added. What Is Acid-Base Buffer System.
From www.studypool.com
SOLUTION Acid base balance buffer system Studypool What Is Acid-Base Buffer System A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. It is able to neutralize small amounts of added acid or. What Is Acid-Base Buffer System.
From labpedia.net
Acidbase Balance Part 1 A Introduction to the AcidBase Balance What Is Acid-Base Buffer System Define buffers and know the composition of different buffer systems. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. One mechanism the body uses to control blood. What Is Acid-Base Buffer System.
From www.studypool.com
SOLUTION Acid base balance buffer system Studypool What Is Acid-Base Buffer System It is able to neutralize small amounts of added acid or base, thus. Define buffers and know the composition of different buffer systems. A buffer is a chemical system that prevents a radical change in fluid ph by dampening the change in hydrogen ion concentrations in the. A buffer is a solution that can resist ph change upon the addition. What Is Acid-Base Buffer System.