Explain How Limescale Form In Kettles Pans And Boilers . Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. Enjoyed this post & graphic? It is a deposit of calcium carbonate and a residue that is left behind by hard. Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. How to remove limescale from your kettle. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard.
from www.alamy.com
Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. How to remove limescale from your kettle. It is a deposit of calcium carbonate and a residue that is left behind by hard. Enjoyed this post & graphic? For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders.
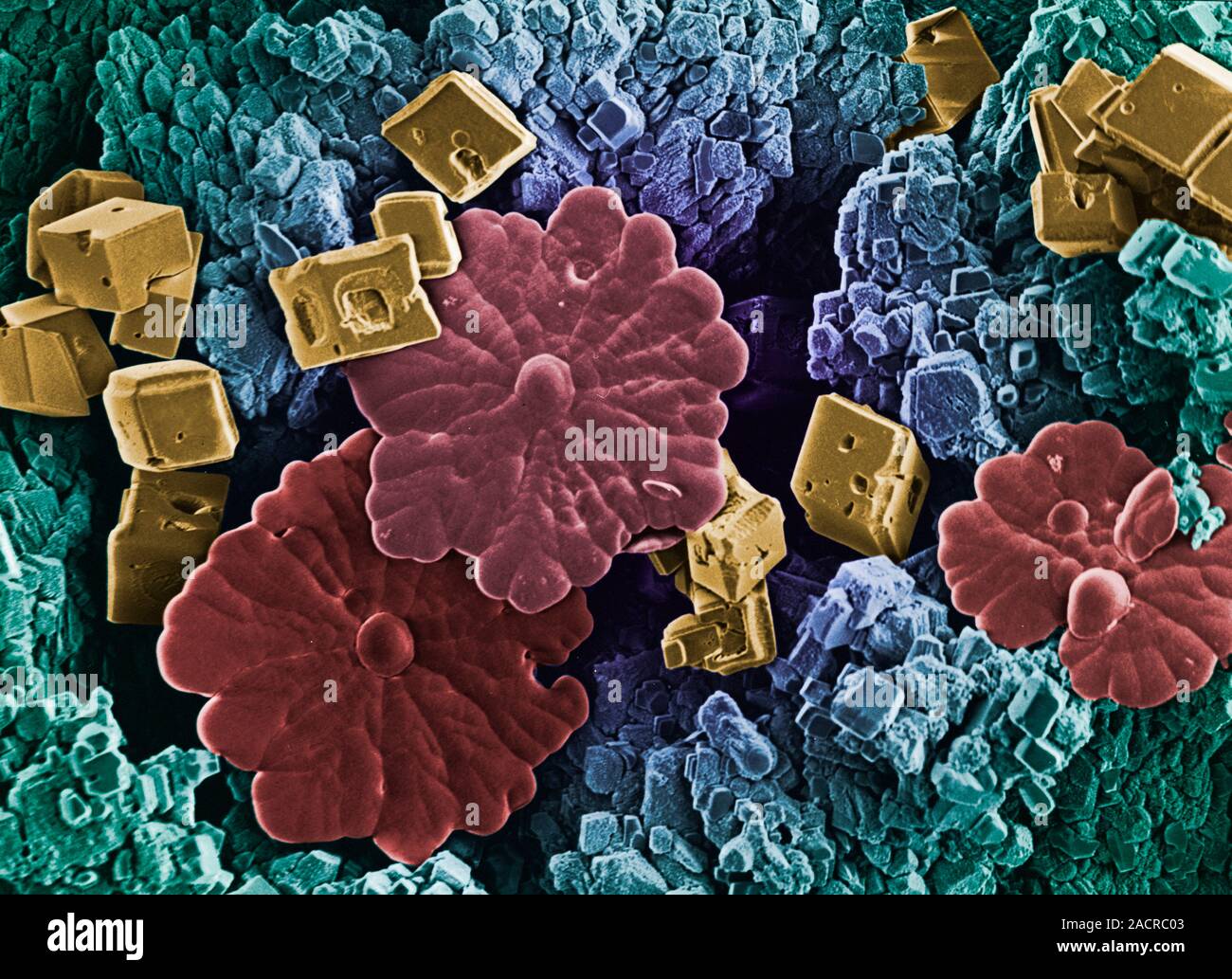
Limescale deposits, from the surface of a water heater. Limescale forms
Explain How Limescale Form In Kettles Pans And Boilers Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. How to remove limescale from your kettle. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. Enjoyed this post & graphic? It is a deposit of calcium carbonate and a residue that is left behind by hard.
From www.slideserve.com
PPT Hardness of Water PowerPoint Presentation ID2956417 Explain How Limescale Form In Kettles Pans And Boilers It is a deposit of calcium carbonate and a residue that is left behind by hard. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. Enjoyed this post & graphic? Limescale is a hard, chalky deposit that forms. Explain How Limescale Form In Kettles Pans And Boilers.
From www.the-sun.com
Mum shares tip to rid kettles of thick limescale without scrubbing Explain How Limescale Form In Kettles Pans And Boilers Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. Limescale buildup in a kettle is caused by the accumulation of. Explain How Limescale Form In Kettles Pans And Boilers.
From slideplayer.com
Autoclave. The autoclave is a piece of equipment used for sterilizing Explain How Limescale Form In Kettles Pans And Boilers How to remove limescale from your kettle. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. It is a deposit of calcium carbonate and a residue that is left behind by hard. Limescale buildup in a kettle is. Explain How Limescale Form In Kettles Pans And Boilers.
From www.youtube.com
How to Remove Limescale from your Kettle YouTube Explain How Limescale Form In Kettles Pans And Boilers Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. How to remove limescale from your kettle. For toilets, stronger acids such as hydrochloric acid are used, whereas for. Explain How Limescale Form In Kettles Pans And Boilers.
From nwmaids.com
How to Remove Limescale and Clean Your Kettle? NW Maids Explain How Limescale Form In Kettles Pans And Boilers How to remove limescale from your kettle. Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders.. Explain How Limescale Form In Kettles Pans And Boilers.
From www.mmcscommercialcleaning.co.uk
How to Remove Limescale from a Kettle Explain How Limescale Form In Kettles Pans And Boilers It is a deposit of calcium carbonate and a residue that is left behind by hard. Enjoyed this post & graphic? Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium. Explain How Limescale Form In Kettles Pans And Boilers.
From www.water-monitoring.com
problems of steam boiler and reverse osmosis OFS Online Fluid Explain How Limescale Form In Kettles Pans And Boilers For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. It is a deposit of calcium carbonate and a residue that is left behind by hard. Limescale buildup in a kettle is caused by the accumulation of minerals such. Explain How Limescale Form In Kettles Pans And Boilers.
From www.completehomefiltration.com.au
Why Should I Care About Limescale? Complete Home Filtration Explain How Limescale Form In Kettles Pans And Boilers Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. Enjoyed this post & graphic? Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium. Explain How Limescale Form In Kettles Pans And Boilers.
From www.express.co.uk
How to remove kettle limescale instantly to make appliance boil ‘so Explain How Limescale Form In Kettles Pans And Boilers Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. Enjoyed this post & graphic? Acids. Explain How Limescale Form In Kettles Pans And Boilers.
From www.alamy.com
Kettle lime scale hires stock photography and images Alamy Explain How Limescale Form In Kettles Pans And Boilers For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. How to remove limescale from your kettle.. Explain How Limescale Form In Kettles Pans And Boilers.
From stock.adobe.com
Limescale, lime scale in old kettle in kitchen. A white, chalky residue Explain How Limescale Form In Kettles Pans And Boilers Enjoyed this post & graphic? How to remove limescale from your kettle. Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common. Explain How Limescale Form In Kettles Pans And Boilers.
From www.express.co.uk
Limescale in kettles ‘increases energy consumption’ ‘highly effective Explain How Limescale Form In Kettles Pans And Boilers Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. It is a deposit of calcium carbonate and a residue that is left behind by. Explain How Limescale Form In Kettles Pans And Boilers.
From medium.com
How to make homemade descaler. Kettles, pipes and boilers, limescale Explain How Limescale Form In Kettles Pans And Boilers Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. It is a deposit of calcium carbonate and a residue that is left behind by hard. How to remove limescale from your kettle. Enjoyed this post & graphic? Limescale buildup in a kettle is caused by the accumulation. Explain How Limescale Form In Kettles Pans And Boilers.
From storage.googleapis.com
How Long Does It Take For Limescale To Build Up Explain How Limescale Form In Kettles Pans And Boilers Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. How to remove limescale from your kettle. Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen. Explain How Limescale Form In Kettles Pans And Boilers.
From www.reddit.com
I wonder if anyone can explain why the limescale in my kettle is blue Explain How Limescale Form In Kettles Pans And Boilers Enjoyed this post & graphic? For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. Acids. Explain How Limescale Form In Kettles Pans And Boilers.
From www.slideserve.com
PPT PM3125 Lectures 13 to 15 PowerPoint Presentation, free download Explain How Limescale Form In Kettles Pans And Boilers Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid,. Explain How Limescale Form In Kettles Pans And Boilers.
From garlicdelight.com
How to clean and descale your kettle to remove limescale Explain How Limescale Form In Kettles Pans And Boilers Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. Enjoyed this post & graphic? It is a deposit of calcium carbonate and a residue that is left behind. Explain How Limescale Form In Kettles Pans And Boilers.
From www.alamy.com
Limescale kettle hires stock photography and images Alamy Explain How Limescale Form In Kettles Pans And Boilers Enjoyed this post & graphic? Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. How. Explain How Limescale Form In Kettles Pans And Boilers.
From garlicdelight.com
How to clean and descale your kettle to remove limescale Explain How Limescale Form In Kettles Pans And Boilers It is a deposit of calcium carbonate and a residue that is left behind by hard. Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. How to remove limescale from your kettle. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances. Explain How Limescale Form In Kettles Pans And Boilers.
From www.express.co.uk
Limescale buildup in kettles can increase ‘running costs’ ‘most Explain How Limescale Form In Kettles Pans And Boilers Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. Enjoyed this post & graphic? Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium. Explain How Limescale Form In Kettles Pans And Boilers.
From garlicdelight.com
How to clean and descale your kettle to remove limescale Explain How Limescale Form In Kettles Pans And Boilers It is a deposit of calcium carbonate and a residue that is left behind by hard. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. Limescale is a hard, chalky deposit that forms when water with high mineral. Explain How Limescale Form In Kettles Pans And Boilers.
From www.stone-stream.com
What is limescale? How to Prevent Limescale Stone Stream Explain How Limescale Form In Kettles Pans And Boilers How to remove limescale from your kettle. Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. Enjoyed this post & graphic? Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. Limescale buildup in a kettle is caused by. Explain How Limescale Form In Kettles Pans And Boilers.
From www.descaler.co.uk
How to How to remove limescale from a Kettle Descaler.co.uk Explain How Limescale Form In Kettles Pans And Boilers For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. It is a deposit of calcium carbonate and a residue that is left behind by hard. Enjoyed this post & graphic? How to remove limescale from your kettle. Limescale. Explain How Limescale Form In Kettles Pans And Boilers.
From www.camelblogs.com
Maintain your boiler to prevent limescale buildup Camel Blogs Explain How Limescale Form In Kettles Pans And Boilers Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. It is a deposit of calcium carbonate and a residue that is left behind by. Explain How Limescale Form In Kettles Pans And Boilers.
From mybudgetrecipes.com
Why Kettles Get Limescale and How To Remove It My Budget Recipes Explain How Limescale Form In Kettles Pans And Boilers Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. Limescale buildup in a kettle is caused by the accumulation of. Explain How Limescale Form In Kettles Pans And Boilers.
From www.numerade.com
SOLVED "Boiler scale" forms in kettles, consisting mainly of calcium Explain How Limescale Form In Kettles Pans And Boilers Enjoyed this post & graphic? For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. How. Explain How Limescale Form In Kettles Pans And Boilers.
From tidyhere.com
Here's How to Clean and Restore Your Kettle TidyHere Explain How Limescale Form In Kettles Pans And Boilers Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. Enjoyed this post & graphic? It is a deposit of calcium carbonate and a residue that is left behind by hard. Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found. Explain How Limescale Form In Kettles Pans And Boilers.
From www.maidforyou.com.au
How to Remove Limescale and Clean Your Kettle MaidForYou Explain How Limescale Form In Kettles Pans And Boilers Enjoyed this post & graphic? It is a deposit of calcium carbonate and a residue that is left behind by hard. Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid,. Explain How Limescale Form In Kettles Pans And Boilers.
From www.pinterest.com
How to clean and descale your kettle to remove limescale Remove Water Explain How Limescale Form In Kettles Pans And Boilers Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. How to remove limescale from your kettle. It is a deposit of calcium carbonate and a residue that is left behind by hard. Enjoyed this post & graphic? For toilets, stronger acids such as hydrochloric acid are used,. Explain How Limescale Form In Kettles Pans And Boilers.
From electricalworkbook.com
What is Steam Jacketed Kettle? Working Principle, Construction, Diagram Explain How Limescale Form In Kettles Pans And Boilers Enjoyed this post & graphic? It is a deposit of calcium carbonate and a residue that is left behind by hard. Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found. Explain How Limescale Form In Kettles Pans And Boilers.
From yourheat.co.uk
How Does Limescale Affect Your Boiler and How to Descale It Your Heat Explain How Limescale Form In Kettles Pans And Boilers Enjoyed this post & graphic? For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. How to remove limescale from your kettle. It is a deposit of calcium carbonate and a residue that is left behind by hard. Acids. Explain How Limescale Form In Kettles Pans And Boilers.
From kitchendary.com
How to Prevent Limescale in Kettle Top 5 Effective Methods Explain How Limescale Form In Kettles Pans And Boilers It is a deposit of calcium carbonate and a residue that is left behind by hard. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. Limescale buildup in a kettle is caused by the accumulation of minerals such. Explain How Limescale Form In Kettles Pans And Boilers.
From www.alamy.com
Limescale deposits, from the surface of a water heater. Limescale forms Explain How Limescale Form In Kettles Pans And Boilers Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. How to remove limescale from your kettle. Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium, which are commonly found in hard. Acids will react with the limescale to produce. Explain How Limescale Form In Kettles Pans And Boilers.
From www.lexairconditioning.com
What is Limescale? Limescale & Its Effect on Plumbing Systems Explain How Limescale Form In Kettles Pans And Boilers It is a deposit of calcium carbonate and a residue that is left behind by hard. Limescale is a hard, chalky deposit that forms when water with high mineral content, particularly calcium and magnesium, evaporates or is heated. Enjoyed this post & graphic? Limescale buildup in a kettle is caused by the accumulation of minerals such as calcium and magnesium,. Explain How Limescale Form In Kettles Pans And Boilers.
From www.youtube.com
How to Remove Limescale from Pans YouTube Explain How Limescale Form In Kettles Pans And Boilers How to remove limescale from your kettle. For toilets, stronger acids such as hydrochloric acid are used, whereas for kitchen appliances such as kettles, citric acid, lactic acid, or formic acid are common components of limescale removal powders. Acids will react with the limescale to produce soluble metal salts which can then simply be washed away. It is a deposit. Explain How Limescale Form In Kettles Pans And Boilers.