Internal Energy Vs Kinetic Energy . Therefore , the change in internal energy is equal to the change in kinetic energy of the system. Internal energy and thermal energy are very similar in a basic thermodynamic context. However, they differ becuase internal energy encompasses far more than just the average. The internal energy of a system is the sum of the kinetic energies and the potential. The work done on the system will. Is internal energy equal to kinetic energy? The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles, as shown in the figure below. From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the object is “hot.” when the. When the sample of water and copper are both heated by 1°c, the addition to the kinetic energy is the same, since that is what temperature.
from www.slideserve.com
Internal energy and thermal energy are very similar in a basic thermodynamic context. Therefore , the change in internal energy is equal to the change in kinetic energy of the system. When the sample of water and copper are both heated by 1°c, the addition to the kinetic energy is the same, since that is what temperature. From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. Is internal energy equal to kinetic energy? The work done on the system will. The internal energy of a system is the sum of the kinetic energies and the potential. The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles, as shown in the figure below. However, they differ becuase internal energy encompasses far more than just the average. When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the object is “hot.” when the.
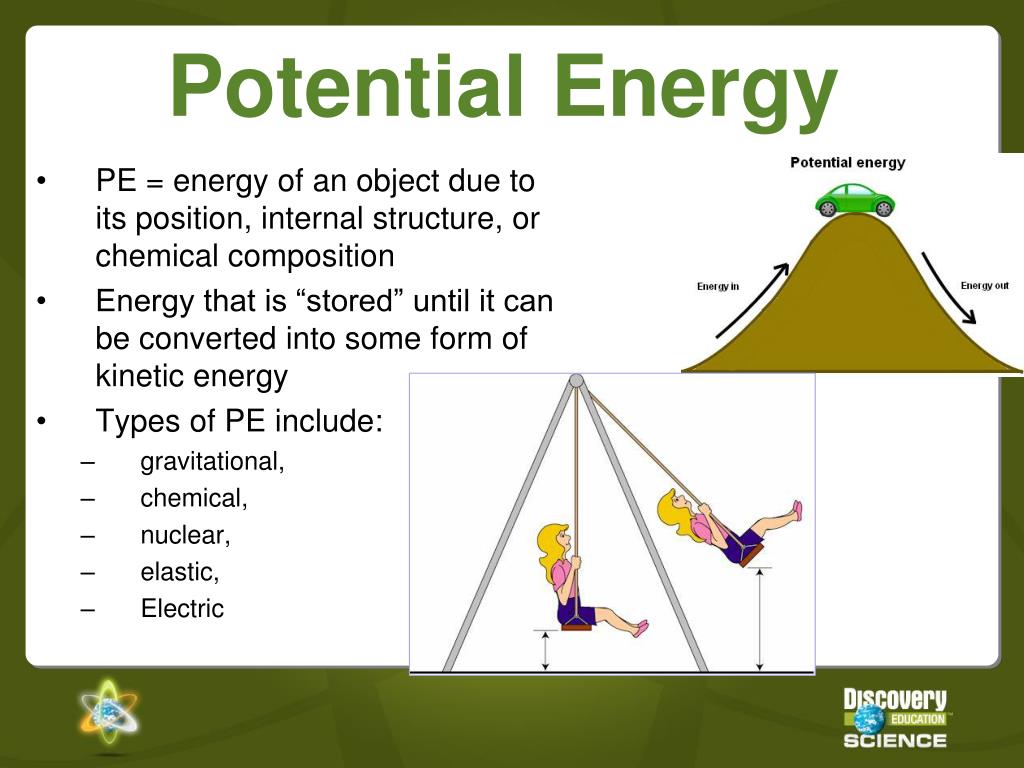
PPT Potential and Energy PowerPoint Presentation, free
Internal Energy Vs Kinetic Energy When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the object is “hot.” when the. The internal energy of a system is the sum of the kinetic energies and the potential. From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. Therefore , the change in internal energy is equal to the change in kinetic energy of the system. When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the object is “hot.” when the. Is internal energy equal to kinetic energy? When the sample of water and copper are both heated by 1°c, the addition to the kinetic energy is the same, since that is what temperature. The work done on the system will. However, they differ becuase internal energy encompasses far more than just the average. The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles, as shown in the figure below. Internal energy and thermal energy are very similar in a basic thermodynamic context.
From www.grc.nasa.gov
First Law of Thermodynamics Internal Energy Vs Kinetic Energy However, they differ becuase internal energy encompasses far more than just the average. When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the object is “hot.” when the. The internal energy of a system is the sum of the kinetic energies and the potential.. Internal Energy Vs Kinetic Energy.
From psiberg.com
Internal Energy vs. Enthalpy Thermodynamics PSIBERG Internal Energy Vs Kinetic Energy The work done on the system will. However, they differ becuase internal energy encompasses far more than just the average. The internal energy of a system is the sum of the kinetic energies and the potential. From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. Is. Internal Energy Vs Kinetic Energy.
From pediaa.com
Difference Between Enthalpy and Internal Energy Definition, Units Internal Energy Vs Kinetic Energy The work done on the system will. When the sample of water and copper are both heated by 1°c, the addition to the kinetic energy is the same, since that is what temperature. The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles, as shown in the figure. Internal Energy Vs Kinetic Energy.
From www.researchgate.net
Conversion of energy and internal energy Download Scientific Internal Energy Vs Kinetic Energy The work done on the system will. From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. Therefore , the change in internal energy is equal to the change in kinetic energy of the system. The kinetic molecular theory assumes that the temperature of a gas is. Internal Energy Vs Kinetic Energy.
From mmerevise.co.uk
Thermal Energy Transfer Questions and Revision MME Internal Energy Vs Kinetic Energy Internal energy and thermal energy are very similar in a basic thermodynamic context. The work done on the system will. Therefore , the change in internal energy is equal to the change in kinetic energy of the system. The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles,. Internal Energy Vs Kinetic Energy.
From www.tes.com
Internal Energy Differentiated Lesson For Trilogy AQA Combined Science Internal Energy Vs Kinetic Energy Therefore , the change in internal energy is equal to the change in kinetic energy of the system. The internal energy of a system is the sum of the kinetic energies and the potential. Is internal energy equal to kinetic energy? Internal energy and thermal energy are very similar in a basic thermodynamic context. The work done on the system. Internal Energy Vs Kinetic Energy.
From www.youtube.com
Theory of Gases and Internal Energy YouTube Internal Energy Vs Kinetic Energy The internal energy of a system is the sum of the kinetic energies and the potential. The work done on the system will. Internal energy and thermal energy are very similar in a basic thermodynamic context. When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say. Internal Energy Vs Kinetic Energy.
From www.researchgate.net
Internal energy, energy and total energy versus time Internal Energy Vs Kinetic Energy However, they differ becuase internal energy encompasses far more than just the average. The internal energy of a system is the sum of the kinetic energies and the potential. Therefore , the change in internal energy is equal to the change in kinetic energy of the system. When the atoms and molecules in an object are moving or vibrating quickly,. Internal Energy Vs Kinetic Energy.
From www.learnatnoon.com
Difference between Potential and Energy Learn At Noon Internal Energy Vs Kinetic Energy The work done on the system will. However, they differ becuase internal energy encompasses far more than just the average. Internal energy and thermal energy are very similar in a basic thermodynamic context. The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles, as shown in the figure. Internal Energy Vs Kinetic Energy.
From www.youtube.com
What is the Difference Between Energy and Potential Energy Internal Energy Vs Kinetic Energy When the sample of water and copper are both heated by 1°c, the addition to the kinetic energy is the same, since that is what temperature. Therefore , the change in internal energy is equal to the change in kinetic energy of the system. Internal energy and thermal energy are very similar in a basic thermodynamic context. The work done. Internal Energy Vs Kinetic Energy.
From online-learning-college.com
Gravitational potential and energy Calculating energies Internal Energy Vs Kinetic Energy The internal energy of a system is the sum of the kinetic energies and the potential. Internal energy and thermal energy are very similar in a basic thermodynamic context. The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles, as shown in the figure below. Therefore , the. Internal Energy Vs Kinetic Energy.
From www.slideserve.com
PPT What are and Potential Energy? PowerPoint Presentation Internal Energy Vs Kinetic Energy From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. The work done on the system will. When the sample of water and copper are both heated by 1°c, the addition to the kinetic energy is the same, since that is what temperature. The kinetic molecular theory. Internal Energy Vs Kinetic Energy.
From www.youtube.com
Potential and energy Law of conservation of energy Video Internal Energy Vs Kinetic Energy The work done on the system will. Is internal energy equal to kinetic energy? From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. Therefore , the change in internal energy is equal to the change in kinetic energy of the system. Internal energy and thermal energy. Internal Energy Vs Kinetic Energy.
From kidsdiscover.com
Infographic Potential vs. Energy Kids Discover Internal Energy Vs Kinetic Energy Internal energy and thermal energy are very similar in a basic thermodynamic context. From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say. Internal Energy Vs Kinetic Energy.
From byjus.com
The average internal energy of molecules of a substance is a Internal Energy Vs Kinetic Energy Therefore , the change in internal energy is equal to the change in kinetic energy of the system. Internal energy and thermal energy are very similar in a basic thermodynamic context. The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles, as shown in the figure below. When. Internal Energy Vs Kinetic Energy.
From whatsinsight.org
energy vs. Potential energy 10 RealLife Examples What's Insight Internal Energy Vs Kinetic Energy Therefore , the change in internal energy is equal to the change in kinetic energy of the system. From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. Internal energy and thermal energy are very similar in a basic thermodynamic context. The kinetic molecular theory assumes that. Internal Energy Vs Kinetic Energy.
From www.careerpower.in
Energy Definition, Example and Derivation Internal Energy Vs Kinetic Energy The work done on the system will. When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the object is “hot.” when the. Is internal energy equal to kinetic energy? The kinetic molecular theory assumes that the temperature of a gas is directly proportional to. Internal Energy Vs Kinetic Energy.
From www.iowalum.com
Middle School Lesson Plan What is Energy? Internal Energy Vs Kinetic Energy The work done on the system will. When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the object is “hot.” when the. However, they differ becuase internal energy encompasses far more than just the average. When the sample of water and copper are both. Internal Energy Vs Kinetic Energy.
From www.researchgate.net
Histories of energy, internal energy, and their ratio of the Internal Energy Vs Kinetic Energy The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles, as shown in the figure below. Therefore , the change in internal energy is equal to the change in kinetic energy of the system. The internal energy of a system is the sum of the kinetic energies and. Internal Energy Vs Kinetic Energy.
From engineerfix.com
What Is Energy? Definition, Examples, Equation, and FAQs Internal Energy Vs Kinetic Energy When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the object is “hot.” when the. The work done on the system will. The internal energy of a system is the sum of the kinetic energies and the potential. However, they differ becuase internal energy. Internal Energy Vs Kinetic Energy.
From www.youtube.com
The Average Energy per Molecule Equation for an Ideal Gas IB Internal Energy Vs Kinetic Energy The work done on the system will. From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. Internal energy and thermal energy are very similar in a basic thermodynamic context. Is internal energy equal to kinetic energy? When the atoms and molecules in an object are moving. Internal Energy Vs Kinetic Energy.
From sciencenotes.org
What Is Energy? Energy Examples Internal Energy Vs Kinetic Energy Therefore , the change in internal energy is equal to the change in kinetic energy of the system. The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles, as shown in the figure below. Internal energy and thermal energy are very similar in a basic thermodynamic context. When. Internal Energy Vs Kinetic Energy.
From www.slideserve.com
PPT Potential and Energy PowerPoint Presentation, free Internal Energy Vs Kinetic Energy Is internal energy equal to kinetic energy? The internal energy of a system is the sum of the kinetic energies and the potential. When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the object is “hot.” when the. The kinetic molecular theory assumes that. Internal Energy Vs Kinetic Energy.
From www.techtarget.com
What is energy? TechTarget Definition Internal Energy Vs Kinetic Energy From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. Internal energy and thermal energy are very similar in a basic thermodynamic context. The internal energy of a system is the sum of the kinetic energies and the potential. The kinetic molecular theory assumes that the temperature. Internal Energy Vs Kinetic Energy.
From eduinput.com
Difference between Energy and Potential Energy Internal Energy Vs Kinetic Energy The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles, as shown in the figure below. When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the object is “hot.” when the. Therefore. Internal Energy Vs Kinetic Energy.
From www.youtube.com
Energy and Potential Energy YouTube Internal Energy Vs Kinetic Energy However, they differ becuase internal energy encompasses far more than just the average. When the sample of water and copper are both heated by 1°c, the addition to the kinetic energy is the same, since that is what temperature. The internal energy of a system is the sum of the kinetic energies and the potential. The kinetic molecular theory assumes. Internal Energy Vs Kinetic Energy.
From www.researchgate.net
Comparison of internal energy versus energy during the FSW at Internal Energy Vs Kinetic Energy The work done on the system will. Internal energy and thermal energy are very similar in a basic thermodynamic context. From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average. Internal Energy Vs Kinetic Energy.
From slideplayer.com
PHYSICS 197 Section 1 Chapter C9 Potential Energy Graphs ppt download Internal Energy Vs Kinetic Energy The work done on the system will. Is internal energy equal to kinetic energy? When the sample of water and copper are both heated by 1°c, the addition to the kinetic energy is the same, since that is what temperature. However, they differ becuase internal energy encompasses far more than just the average. Therefore , the change in internal energy. Internal Energy Vs Kinetic Energy.
From www.showme.com
Physics Potential, and Internal energy Science, Physics Internal Energy Vs Kinetic Energy The work done on the system will. Is internal energy equal to kinetic energy? From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we. Internal Energy Vs Kinetic Energy.
From www.linquip.com
Difference Between Energy and Potential Energy A Clear Guide Internal Energy Vs Kinetic Energy From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. Therefore , the change in internal energy is equal to the change in kinetic energy of the system. When the sample of water and copper are both heated by 1°c, the addition to the kinetic energy is. Internal Energy Vs Kinetic Energy.
From www.youtube.com
Internal Energy in Physics YouTube Internal Energy Vs Kinetic Energy Therefore , the change in internal energy is equal to the change in kinetic energy of the system. The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles, as shown in the figure below. However, they differ becuase internal energy encompasses far more than just the average. Is. Internal Energy Vs Kinetic Energy.
From polarpedia.eu
energi Polarpedia Internal Energy Vs Kinetic Energy However, they differ becuase internal energy encompasses far more than just the average. The work done on the system will. When the sample of water and copper are both heated by 1°c, the addition to the kinetic energy is the same, since that is what temperature. Therefore , the change in internal energy is equal to the change in kinetic. Internal Energy Vs Kinetic Energy.
From www.youtube.com
and Potential Energy Simplified YouTube Internal Energy Vs Kinetic Energy The work done on the system will. Therefore , the change in internal energy is equal to the change in kinetic energy of the system. When the sample of water and copper are both heated by 1°c, the addition to the kinetic energy is the same, since that is what temperature. Internal energy and thermal energy are very similar in. Internal Energy Vs Kinetic Energy.
From differencebtw.com
Energy vs. Potential Energy Know the Difference Internal Energy Vs Kinetic Energy From the point of view of mechanics, the internal energy is then the value of total energy of the system (kinetic+potential) in this. Therefore , the change in internal energy is equal to the change in kinetic energy of the system. The work done on the system will. Is internal energy equal to kinetic energy? Internal energy and thermal energy. Internal Energy Vs Kinetic Energy.
From www.etutorworld.com
and Potential Energy Definitions, Key Difference, Examples and Internal Energy Vs Kinetic Energy When the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the object is “hot.” when the. Internal energy and thermal energy are very similar in a basic thermodynamic context. The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the. Internal Energy Vs Kinetic Energy.