What Makes A Solvent Pair Too Good . in the event that a solid is too soluble in one solvent, but too insoluble in a second solvent, a mixed solvent system (solvent. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). If the sample does not. In most cases, the organic solvent is not. when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. if the sample dissolves completely, the solubility in the cold solvent is too high to be a good recrystallization solvent. It is also important that the. So we can not use the ethanol as a solvent for. if the substance is found to be far too soluble in one solvent and much too insoluble in another solvent to allow of satisfactory. acetanilide is readily soluble in ethanol at room temperature. an ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity.
from www.chegg.com
It is also important that the. when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. an ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. acetanilide is readily soluble in ethanol at room temperature. if the substance is found to be far too soluble in one solvent and much too insoluble in another solvent to allow of satisfactory. So we can not use the ethanol as a solvent for. if the sample dissolves completely, the solubility in the cold solvent is too high to be a good recrystallization solvent. in the event that a solid is too soluble in one solvent, but too insoluble in a second solvent, a mixed solvent system (solvent. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). In most cases, the organic solvent is not.
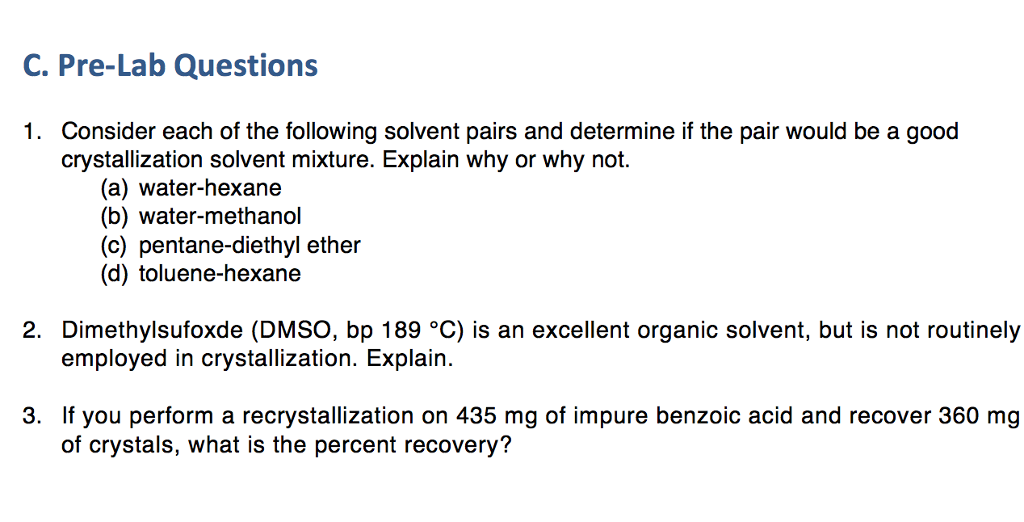
Solved C. PreLab Questions Consider each of the following
What Makes A Solvent Pair Too Good So we can not use the ethanol as a solvent for. In most cases, the organic solvent is not. So we can not use the ethanol as a solvent for. acetanilide is readily soluble in ethanol at room temperature. in the event that a solid is too soluble in one solvent, but too insoluble in a second solvent, a mixed solvent system (solvent. when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. if the substance is found to be far too soluble in one solvent and much too insoluble in another solvent to allow of satisfactory. It is also important that the. an ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). if the sample dissolves completely, the solubility in the cold solvent is too high to be a good recrystallization solvent. If the sample does not.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID375520 What Makes A Solvent Pair Too Good In most cases, the organic solvent is not. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). It is also important that the. if the sample dissolves completely, the solubility in the cold solvent is too high to be a good recrystallization solvent. when no single solvent. What Makes A Solvent Pair Too Good.
From gbu-presnenskij.ru
Solvent Definition, Types, Incredible Uses, Examples, 56 OFF What Makes A Solvent Pair Too Good It is also important that the. in the event that a solid is too soluble in one solvent, but too insoluble in a second solvent, a mixed solvent system (solvent. acetanilide is readily soluble in ethanol at room temperature. In most cases, the organic solvent is not. If the sample does not. an ideal crystallization solvent should. What Makes A Solvent Pair Too Good.
From www.studypug.com
Introduction to Solution Chemistry Explore Solubility StudyPug What Makes A Solvent Pair Too Good if the substance is found to be far too soluble in one solvent and much too insoluble in another solvent to allow of satisfactory. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). in the event that a solid is too soluble in one solvent, but too. What Makes A Solvent Pair Too Good.
From www.numerade.com
SOLVED Important criteria for selecting recrystallization solvent 1 What Makes A Solvent Pair Too Good when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). acetanilide is readily soluble in ethanol at room temperature. an ideal crystallization solvent. What Makes A Solvent Pair Too Good.
From exoponlbe.blob.core.windows.net
What Makes A Good Solvent at Reginald Pearson blog What Makes A Solvent Pair Too Good an ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. So we can not use the ethanol as a solvent for. a solvent partitioning almost always involves the use of water. What Makes A Solvent Pair Too Good.
From www.pinterest.com
Choosing the Solvent and Solvent Pairs Solvent, I.n hot, Temperatures What Makes A Solvent Pair Too Good an ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. if the substance is found to be far too soluble in one solvent and much too insoluble in another solvent to allow of satisfactory. acetanilide is readily soluble in ethanol at room temperature. So we can not use the ethanol as a solvent for. In. What Makes A Solvent Pair Too Good.
From www.masterorganicchemistry.com
What Makes A Good Nucleophile? Master Organic Chemistry What Makes A Solvent Pair Too Good acetanilide is readily soluble in ethanol at room temperature. when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. In most cases, the organic solvent is not. So we can not use the ethanol as a solvent for. in the event that a. What Makes A Solvent Pair Too Good.
From www.masterorganicchemistry.com
All about Solvents NonPolar, Polar Aprotic, and Polar Protic Solvents What Makes A Solvent Pair Too Good in the event that a solid is too soluble in one solvent, but too insoluble in a second solvent, a mixed solvent system (solvent. It is also important that the. an ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. If the sample does not. a solvent partitioning almost always involves the use of water. What Makes A Solvent Pair Too Good.
From www.masterorganicchemistry.com
What Makes A Good Nucleophile? Master Organic Chemistry What Makes A Solvent Pair Too Good If the sample does not. if the substance is found to be far too soluble in one solvent and much too insoluble in another solvent to allow of satisfactory. So we can not use the ethanol as a solvent for. if the sample dissolves completely, the solubility in the cold solvent is too high to be a good. What Makes A Solvent Pair Too Good.
From www.youtube.com
Solutes, Solvents and Solutions REMOTE 1 YouTube What Makes A Solvent Pair Too Good a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). acetanilide is readily soluble in ethanol at room temperature. So we can not use the ethanol as a solvent for. when no single solvent can be found that meets all of the criteria for crystallization, it may be. What Makes A Solvent Pair Too Good.
From www.youtube.com
Recrystallization Choosing Solvent & Inducing Crystallization YouTube What Makes A Solvent Pair Too Good If the sample does not. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). acetanilide is readily soluble in ethanol at room temperature. when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent.. What Makes A Solvent Pair Too Good.
From exoponlbe.blob.core.windows.net
What Makes A Good Solvent at Reginald Pearson blog What Makes A Solvent Pair Too Good a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). in the event that a solid is too soluble in one solvent, but too insoluble in a second solvent, a mixed solvent system (solvent. an ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. So we. What Makes A Solvent Pair Too Good.
From www.youtube.com
Solute, solvent and solution What is a Solution? Science Video for What Makes A Solvent Pair Too Good in the event that a solid is too soluble in one solvent, but too insoluble in a second solvent, a mixed solvent system (solvent. It is also important that the. when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. acetanilide is readily. What Makes A Solvent Pair Too Good.
From classlibabend.z21.web.core.windows.net
Water The Universal Solvent Worksheet What Makes A Solvent Pair Too Good if the sample dissolves completely, the solubility in the cold solvent is too high to be a good recrystallization solvent. when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. a solvent partitioning almost always involves the use of water and an organic. What Makes A Solvent Pair Too Good.
From exoponlbe.blob.core.windows.net
What Makes A Good Solvent at Reginald Pearson blog What Makes A Solvent Pair Too Good if the substance is found to be far too soluble in one solvent and much too insoluble in another solvent to allow of satisfactory. It is also important that the. when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. In most cases, the. What Makes A Solvent Pair Too Good.
From www.woodsmith.com
A Guide To Finishing Solvents Woodsmith What Makes A Solvent Pair Too Good acetanilide is readily soluble in ethanol at room temperature. So we can not use the ethanol as a solvent for. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). when no single solvent can be found that meets all of the criteria for crystallization, it may be. What Makes A Solvent Pair Too Good.
From www.chegg.com
Solved C. PreLab Questions Consider each of the following What Makes A Solvent Pair Too Good If the sample does not. It is also important that the. In most cases, the organic solvent is not. if the substance is found to be far too soluble in one solvent and much too insoluble in another solvent to allow of satisfactory. when no single solvent can be found that meets all of the criteria for crystallization,. What Makes A Solvent Pair Too Good.
From thechemistrynotes.com
Solvent Definition, Types, Incredible Uses, Examples What Makes A Solvent Pair Too Good in the event that a solid is too soluble in one solvent, but too insoluble in a second solvent, a mixed solvent system (solvent. an ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). If the. What Makes A Solvent Pair Too Good.
From www.pinterest.com.mx
Solubility Rules Chart for Chemistry Classroom Teaching chemistry What Makes A Solvent Pair Too Good If the sample does not. acetanilide is readily soluble in ethanol at room temperature. an ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). in the event that a solid is too soluble in one. What Makes A Solvent Pair Too Good.
From www.youtube.com
Difference between solute and solvent YouTube What Makes A Solvent Pair Too Good In most cases, the organic solvent is not. It is also important that the. if the sample dissolves completely, the solubility in the cold solvent is too high to be a good recrystallization solvent. acetanilide is readily soluble in ethanol at room temperature. So we can not use the ethanol as a solvent for. in the event. What Makes A Solvent Pair Too Good.
From www.masterorganicchemistry.com
What Makes A Good Nucleophile? Master Organic Chemistry What Makes A Solvent Pair Too Good in the event that a solid is too soluble in one solvent, but too insoluble in a second solvent, a mixed solvent system (solvent. So we can not use the ethanol as a solvent for. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). if the substance. What Makes A Solvent Pair Too Good.
From dxoxozfxr.blob.core.windows.net
Examples Of Solvents And Solutes at Milton Nelson blog What Makes A Solvent Pair Too Good It is also important that the. If the sample does not. In most cases, the organic solvent is not. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). an ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. if the sample dissolves completely, the solubility. What Makes A Solvent Pair Too Good.
From www.sciencephoto.com
Household solvents Stock Image H130/0358 Science Photo Library What Makes A Solvent Pair Too Good when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. So we can not use the ethanol as a solvent for. in the event that a solid is too soluble in one solvent, but too insoluble in a second solvent, a mixed solvent system. What Makes A Solvent Pair Too Good.
From www.masterorganicchemistry.com
All about Solvents NonPolar, Polar Aprotic, and Polar Protic Solvents What Makes A Solvent Pair Too Good So we can not use the ethanol as a solvent for. It is also important that the. an ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. if the substance is found to be far too soluble in one solvent and much too insoluble in another solvent to allow of satisfactory. If the sample does not.. What Makes A Solvent Pair Too Good.
From chem.libretexts.org
3.1 What is a Solvent? Chemistry LibreTexts What Makes A Solvent Pair Too Good in the event that a solid is too soluble in one solvent, but too insoluble in a second solvent, a mixed solvent system (solvent. It is also important that the. when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. if the substance. What Makes A Solvent Pair Too Good.
From draw-nugget.blogspot.com
Polar And Nonpolar Solvents Examples Drawnugget What Makes A Solvent Pair Too Good If the sample does not. acetanilide is readily soluble in ethanol at room temperature. It is also important that the. if the substance is found to be far too soluble in one solvent and much too insoluble in another solvent to allow of satisfactory. So we can not use the ethanol as a solvent for. when no. What Makes A Solvent Pair Too Good.
From dxoxcorli.blob.core.windows.net
What Are Examples Of Non Polar Solvents at Paula West blog What Makes A Solvent Pair Too Good in the event that a solid is too soluble in one solvent, but too insoluble in a second solvent, a mixed solvent system (solvent. In most cases, the organic solvent is not. If the sample does not. if the sample dissolves completely, the solubility in the cold solvent is too high to be a good recrystallization solvent. It. What Makes A Solvent Pair Too Good.
From materialfullchaffrons.z21.web.core.windows.net
Solute In Science What Makes A Solvent Pair Too Good if the substance is found to be far too soluble in one solvent and much too insoluble in another solvent to allow of satisfactory. an ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). In most. What Makes A Solvent Pair Too Good.
From chemawareness.blogspot.com
Chem Awareness Classification of Solvent`s Types of Solvents Examples, What Makes A Solvent Pair Too Good If the sample does not. acetanilide is readily soluble in ethanol at room temperature. when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. In most cases, the organic solvent is not. So we can not use the ethanol as a solvent for. . What Makes A Solvent Pair Too Good.
From www.slideserve.com
PPT Solvents, Recrystallization, and Melting Point PowerPoint What Makes A Solvent Pair Too Good In most cases, the organic solvent is not. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). if the sample dissolves completely, the solubility in the cold solvent is too high to be a good recrystallization solvent. when no single solvent can be found that meets all. What Makes A Solvent Pair Too Good.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID269800 What Makes A Solvent Pair Too Good when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. an ideal crystallization solvent should be unreactive, inexpensive, and have low toxicity. in the event that a solid is too soluble in one solvent, but too insoluble in a second solvent, a mixed. What Makes A Solvent Pair Too Good.
From www.caymanchem.com
Solubility Rules Chart.png What Makes A Solvent Pair Too Good if the sample dissolves completely, the solubility in the cold solvent is too high to be a good recrystallization solvent. It is also important that the. So we can not use the ethanol as a solvent for. in the event that a solid is too soluble in one solvent, but too insoluble in a second solvent, a mixed. What Makes A Solvent Pair Too Good.
From www.researchgate.net
Equation constants for various polymer solvent pairs [17]. Download What Makes A Solvent Pair Too Good if the substance is found to be far too soluble in one solvent and much too insoluble in another solvent to allow of satisfactory. when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. So we can not use the ethanol as a solvent. What Makes A Solvent Pair Too Good.
From www.masterorganicchemistry.com
All about Solvents NonPolar, Polar Aprotic, and Polar Protic Solvents What Makes A Solvent Pair Too Good So we can not use the ethanol as a solvent for. if the substance is found to be far too soluble in one solvent and much too insoluble in another solvent to allow of satisfactory. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). when no single. What Makes A Solvent Pair Too Good.
From www.slideserve.com
PPT Today PowerPoint Presentation, free download ID3856962 What Makes A Solvent Pair Too Good In most cases, the organic solvent is not. when no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. a solvent partitioning almost always involves the use of water and an organic solvent (based on carbon & hydrogen). So we can not use the ethanol. What Makes A Solvent Pair Too Good.