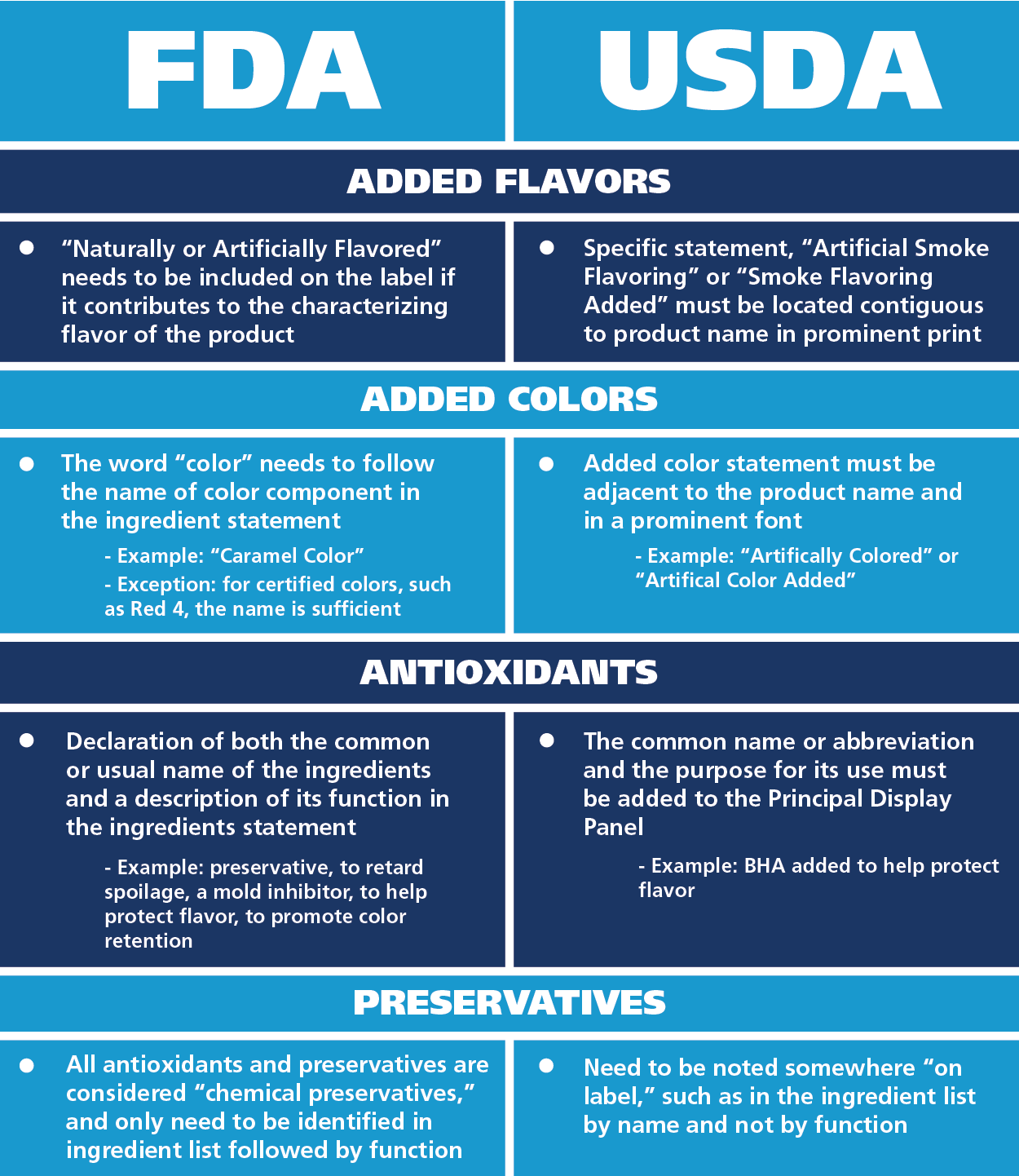
Fda Label Regulations . In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Nutrition labeling for raw produce (fruits and. Introduction to medical device labeling label vs. Food and drug administration (fda) develops and administers regulations under. Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. (1) any person who causes a label to be. § 1.20 presence of mandatory label information.
from regulationlatest.blogspot.com
(1) any person who causes a label to be. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. Introduction to medical device labeling label vs. Food and drug administration (fda) develops and administers regulations under. Nutrition labeling for raw produce (fruits and. § 1.20 presence of mandatory label information. Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc.
Fda Regulations For Food Manufacturing
Fda Label Regulations Nutrition labeling for raw produce (fruits and. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Food and drug administration (fda) develops and administers regulations under. (1) any person who causes a label to be. § 1.20 presence of mandatory label information. Nutrition labeling for raw produce (fruits and. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. Introduction to medical device labeling label vs.
From www.youtube.com
New U.S. FDA Food Labeling Rules YouTube Fda Label Regulations § 1.20 presence of mandatory label information. (1) any person who causes a label to be. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Food and drug administration (fda) develops and administers regulations under. Introduction to medical device labeling label vs. Labeling and packaging materials shall be representatively sampled, and. Fda Label Regulations.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Fda Label Regulations Nutrition labeling for raw produce (fruits and. (1) any person who causes a label to be. Introduction to medical device labeling label vs. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. § 1.20 presence of mandatory label information. In the regulations specified in § 1.1 (c) of this chapter, the. Fda Label Regulations.
From regulationlatest.blogspot.com
Fda Regulations For Food Manufacturing Fda Label Regulations § 1.20 presence of mandatory label information. Food and drug administration (fda) develops and administers regulations under. (1) any person who causes a label to be. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before. Fda Label Regulations.
From www.nbcnews.com
FDA's New Food Labels What to Know NBC News Fda Label Regulations In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. § 1.20 presence of mandatory label information. Introduction to medical device labeling label vs. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. (1) any person who causes a label to be. Food. Fda Label Regulations.
From packaginghub.com
FDA Labeling Requirements For Cosmetics Packaging Hub Fda Label Regulations § 1.20 presence of mandatory label information. Introduction to medical device labeling label vs. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Food and drug administration (fda) develops and administers. Fda Label Regulations.
From www.fda.gov
Guidance for Industry Guide for Developing and Using Data Bases for Fda Label Regulations Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. Food and drug administration (fda) develops and administers regulations under. Introduction to medical device labeling label vs. (1) any person who causes. Fda Label Regulations.
From www.menusano.com
How to Create a FDA Approved Nutrition Fact Label MenuSano Fda Label Regulations Nutrition labeling for raw produce (fruits and. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Label has the meaning set forth in section 201 (k) of the federal food, drug,. Fda Label Regulations.
From cehrjpce.blob.core.windows.net
Fda Label Examples at Claude Patterson blog Fda Label Regulations Food and drug administration (fda) develops and administers regulations under. Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. (1) any person who causes a label to be. Introduction to medical device labeling label vs. In the regulations specified in § 1.1 (c) of this chapter, the term package. Fda Label Regulations.
From www.greenlight.guru
FDA Medical Device Labeling Requirements An Overview Fda Label Regulations Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. § 1.20 presence of mandatory label information. Introduction to medical device labeling label vs. (1) any person who causes a label to be. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic. Fda Label Regulations.
From animalia-life.club
Fda Drug Labeling Requirements Fda Label Regulations Food and drug administration (fda) develops and administers regulations under. Nutrition labeling for raw produce (fruits and. § 1.20 presence of mandatory label information. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act.. Fda Label Regulations.
From ceajakok.blob.core.windows.net
Fda Food Labeling Guide Ingredients at Leroy Johnson blog Fda Label Regulations Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. (1) any person who causes a label to be. Nutrition labeling for raw produce (fruits and. Labeling and packaging materials shall be. Fda Label Regulations.
From www.fdalisting.com
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements Fda Label Regulations Introduction to medical device labeling label vs. Nutrition labeling for raw produce (fruits and. (1) any person who causes a label to be. Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic. Fda Label Regulations.
From ar.inspiredpencil.com
Fda Labeling Regulations Fda Label Regulations Introduction to medical device labeling label vs. Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. § 1.20 presence of mandatory label information. Labeling and packaging materials shall be representatively sampled,. Fda Label Regulations.
From www.fdalisting.com
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements Fda Label Regulations Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Food and drug administration (fda) develops and administers regulations under. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. § 1.20 presence of mandatory label information. Introduction to medical device labeling. Fda Label Regulations.
From klanibqvc.blob.core.windows.net
Fda Regulations For Off Label Use at Kristine Corbin blog Fda Label Regulations Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Food and drug administration (fda) develops and administers regulations under. Label has the meaning set forth in section 201 (k) of the. Fda Label Regulations.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Fda Label Regulations Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. Nutrition labeling for raw produce (fruits and. In the regulations specified in § 1.1 (c) of this chapter, the term package means. Fda Label Regulations.
From www.wmdt.com
FDA makes new rules for labels 47abc Fda Label Regulations In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. (1) any person who causes a label to be. Food and drug administration (fda) develops and administers regulations under. Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. Label has the. Fda Label Regulations.
From nuffoodsspectrum.in
FSSAI takes on new labelling and display regulations FFOODS Spectrum Fda Label Regulations Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Introduction to medical device labeling label vs. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. Nutrition labeling for raw produce (fruits and. § 1.20 presence of mandatory label information. Labeling. Fda Label Regulations.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Fda Label Regulations In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Introduction to medical device labeling label vs. Nutrition labeling for raw produce (fruits and. (1) any person who causes a label to be. Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks,. Fda Label Regulations.
From ar.inspiredpencil.com
Fda Labeling Regulations Fda Label Regulations Food and drug administration (fda) develops and administers regulations under. § 1.20 presence of mandatory label information. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce. Fda Label Regulations.
From labelcalc.com
2020 FDA Regulations for Food Labeling LabelCalc Fda Label Regulations Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. (1) any person who causes a label to be. Introduction to medical device labeling label vs. Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. In the regulations specified. Fda Label Regulations.
From ar.inspiredpencil.com
Fda Labeling Requirements For Food Fda Label Regulations Nutrition labeling for raw produce (fruits and. Introduction to medical device labeling label vs. Food and drug administration (fda) develops and administers regulations under. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act.. Fda Label Regulations.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Label Regulations In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. § 1.20 presence of mandatory label information. (1) any person who causes a label to be. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. Labeling and packaging materials shall be representatively sampled,. Fda Label Regulations.
From www.greenlight.guru
FDA Labeling Requirements Checklist Free Download Fda Label Regulations Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. (1) any person who causes a label to be. § 1.20 presence of mandatory label information. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. In the regulations specified in §. Fda Label Regulations.
From regulationlatest.blogspot.com
Fda Regulations For Food Additives Fda Label Regulations Introduction to medical device labeling label vs. Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. (1) any person who causes a label to be. Label has the meaning. Fda Label Regulations.
From packaginghub.com
FDA Packaging and Labeling Requirements Guide Packaging Hub Fda Label Regulations Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Food and drug administration (fda) develops and administers regulations under. Nutrition labeling for raw produce (fruits and. § 1.20 presence of mandatory label information.. Fda Label Regulations.
From ar.inspiredpencil.com
Fda Labeling Regulations Fda Label Regulations § 1.20 presence of mandatory label information. Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Introduction to medical device labeling label vs. Nutrition labeling for raw produce (fruits. Fda Label Regulations.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Fda Label Regulations (1) any person who causes a label to be. § 1.20 presence of mandatory label information. Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container. Fda Label Regulations.
From nutrition.ftempo.com
Fda Daily Nutritional Requirements Chart Nutrition Ftempo Fda Label Regulations Introduction to medical device labeling label vs. (1) any person who causes a label to be. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. Nutrition labeling for raw produce (fruits. Fda Label Regulations.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Label Regulations (1) any person who causes a label to be. Introduction to medical device labeling label vs. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. Nutrition labeling for raw produce (fruits and. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Food. Fda Label Regulations.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Supplements Fda Label Regulations (1) any person who causes a label to be. Food and drug administration (fda) develops and administers regulations under. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. § 1.20 presence of mandatory label information. Introduction to medical device labeling label vs. Labeling and packaging materials shall be representatively sampled, and. Fda Label Regulations.
From mungfali.com
Food Labeling Fda Label Regulations Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Introduction to medical device labeling label vs. § 1.20 presence of mandatory label information. Nutrition labeling for raw produce (fruits and. Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging. Fda Label Regulations.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Label Regulations Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. Nutrition labeling for raw produce (fruits and. Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. In the regulations specified in § 1.1 (c) of this chapter, the term package means. Fda Label Regulations.
From animalia-life.club
Fda Drug Labeling Requirements Fda Label Regulations Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. Nutrition labeling for raw produce (fruits and. In the regulations specified in § 1.1 (c) of this chapter, the term package means any container or. Introduction to medical device labeling label vs. Label has the meaning set forth in section. Fda Label Regulations.
From animalia-life.club
Fda Drug Labeling Requirements Fda Label Regulations Introduction to medical device labeling label vs. Label has the meaning set forth in section 201 (k) of the federal food, drug, and cosmetic act. Labeling and packaging materials shall be representatively sampled, and examined or tested upon receipt and before use in packaging or. (1) any person who causes a label to be. Food labeling is required for most. Fda Label Regulations.