If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen . For 58g of c 4h 10208go2 is. Closed oct 25, 2021 by hanitasahu. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58 gm of c 4h 10 208gm o2 is.
from www.numerade.com
C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. Closed oct 25, 2021 by hanitasahu. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58 gm of c 4h 10 208gm o2 is. For 58g of c 4h 10208go2 is.
SOLVED 79 Butane exists as two isomers nbutane and isobutane CH3 CHz
If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58 gm of c 4h 10 208gm o2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For 58g of c 4h 10208go2 is. For 58 gm of c 4h 10 208gm o2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. Closed oct 25, 2021 by hanitasahu.
From www.slideserve.com
PPT Organic Compounds PowerPoint Presentation, free download ID2192141 If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58 gm of c 4h 10 208gm o2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For 58g of c 4h 10208go2 is.. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.coursehero.com
[Solved] nButane is converted to isobutane in a continuous If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen Closed oct 25, 2021 by hanitasahu. C 4h 10 + 213o2 → 4c o2 +5h 2o. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58g of c 4h 10208go2 is. For 58 gm of c 4h 10 208gm o2 is. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.doubtnut.com
If isobutane and nbutane are present in a gas, then how much oxygen s If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58g of c 4h 10208go2 is. For 58 gm of c 4h 10 208gm o2 is. Closed oct 25, 2021 by hanitasahu. C 4h 10 + 213o2 → 4c o2 +5h 2o. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.toppr.com
If isobutane and n butane are present in a gas, then how much oxygen If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58 gm of c 4h 10 208gm o2 is. Closed oct 25, 2021 by hanitasahu. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58g of c 4h 10208go2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.numerade.com
SOLVED We have a gaseous sample where isobutane and nbutane are in If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen C 4h 10 + 213o2 → 4c o2 +5h 2o. C 4h 10 + 213o2 → 4c o2 +5h 2o. Closed oct 25, 2021 by hanitasahu. For 58g of c 4h 10208go2 is. For 58 gm of c 4h 10 208gm o2 is. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From byjus.com
If isobutane and n butane are present in a gas , then how much oxygen If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen Closed oct 25, 2021 by hanitasahu. For 58 gm of c 4h 10 208gm o2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58g of c 4h 10208go2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.doubtnut.com
If isobutane and nbutane are present in a gas, then how much oxygen s If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58 gm of c 4h 10 208gm o2 is. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For 58g of c 4h 10208go2 is. Closed oct 25, 2021 by hanitasahu. C 4h 10 +. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.doubtnut.com
If isobutane and nbutane are present in a gas, then how much oxygen s If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58 gm of c 4h 10 208gm o2 is. For 58g of c 4h 10208go2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208.. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.youtube.com
nButane and isobutane are examples of _____. YouTube If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58 gm of c 4h 10 208gm o2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. C 4h 10 + 213o2 → 4c o2 +5h 2o. Closed oct 25, 2021 by hanitasahu. For 58g of c 4h 10208go2 is. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.numerade.com
SOLVED 79 Butane exists as two isomers nbutane and isobutane CH3 CHz If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58 gm of c 4h 10 208gm o2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. Closed oct 25, 2021 by hanitasahu. For. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.chegg.com
Normal butane is to be isomerized to isobutane in a If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58g of c 4h 10208go2 is. For 58 gm of c 4h 10 208gm o2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. C 4h 10 + 213o2 → 4c o2 +5h 2o.. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.taiyugas.com
Isobutane Refrigerant If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For 58 gm of c 4h 10 208gm o2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58g of c 4h 10208go2 is.. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From byjus.com
if LPG cylinder contain mixture of butane and isobutane then the amount If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58g of c 4h 10208go2 is. For 58 gm of c 4h 10 208gm o2 is. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. C 4h 10 + 213o2 → 4c o2 +5h 2o. C 4h 10 + 213o2 → 4c o2 +5h 2o.. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.toppr.com
If isobutane and n butane are present in a gas, then how much oxygen If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58 gm of c 4h 10 208gm o2 is. For 58g of c 4h 10208go2 is. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. C 4h 10 + 213o2 → 4c o2 +5h 2o.. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.coursehero.com
[Solved] nButane is converted to isobutane in a continuous If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58 gm of c 4h 10 208gm o2 is. For 58g of c 4h 10208go2 is. Closed oct 25, 2021 by hanitasahu. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. C 4h 10 +. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.coursehero.com
[Solved] nButane is converted to isobutane in a continuous If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen C 4h 10 + 213o2 → 4c o2 +5h 2o. Closed oct 25, 2021 by hanitasahu. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For 58g of c 4h 10208go2 is. For 58 gm of. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.researchgate.net
Isomerisation mechanisms of n and isobutane. Download Scientific Diagram If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58 gm of c 4h 10 208gm o2 is. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. C 4h 10 + 213o2 → 4c o2 +5h 2o. Closed oct 25, 2021 by hanitasahu. C 4h 10 + 213o2 → 4c o2 +5h 2o. For. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From mungfali.com
Isobutane Phase Diagram If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen Closed oct 25, 2021 by hanitasahu. For 58 gm of c 4h 10 208gm o2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.chegg.com
Solved The structural formulas of the compounds nbutane and If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58 gm of c 4h 10 208gm o2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58g of c 4h 10208go2 is. Closed oct 25, 2021 by hanitasahu. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From brainly.in
draw the structure of N butane and isobutane Brainly.in If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58g of c 4h 10208go2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For 58 gm of c 4h 10 208gm o2 is.. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.researchgate.net
Chemical equilibrium for butane/isobutane gas mixtures, using data from If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58g of c 4h 10208go2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58 gm of c 4h 10 208gm o2 is. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208.. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From pediaa.com
Difference Between Butane and Isobutane Definition, Properties, and If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen Closed oct 25, 2021 by hanitasahu. For 58 gm of c 4h 10 208gm o2 is. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. C 4h 10 + 213o2 → 4c o2 +5h 2o. C 4h 10 + 213o2 → 4c o2 +5h 2o. For. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.ranksbooster.in
If isobutane and nbutane are present in a gas, then how much oxygen If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen C 4h 10 + 213o2 → 4c o2 +5h 2o. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58 gm of c 4h 10 208gm o2 is. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For 58g of c 4h 10208go2 is.. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.coursehero.com
[Solved] nButane is converted to isobutane in a continuous If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen Closed oct 25, 2021 by hanitasahu. For 58 gm of c 4h 10 208gm o2 is. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For 58g of c 4h 10208go2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. C 4h 10 +. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.chegg.com
Solved The catalytic isomerization of nbutane to isobutane If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For 58 gm of c 4h 10 208gm o2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58g of c 4h 10208go2 is. Closed oct 25, 2021 by hanitasahu. C 4h 10 +. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.coursehero.com
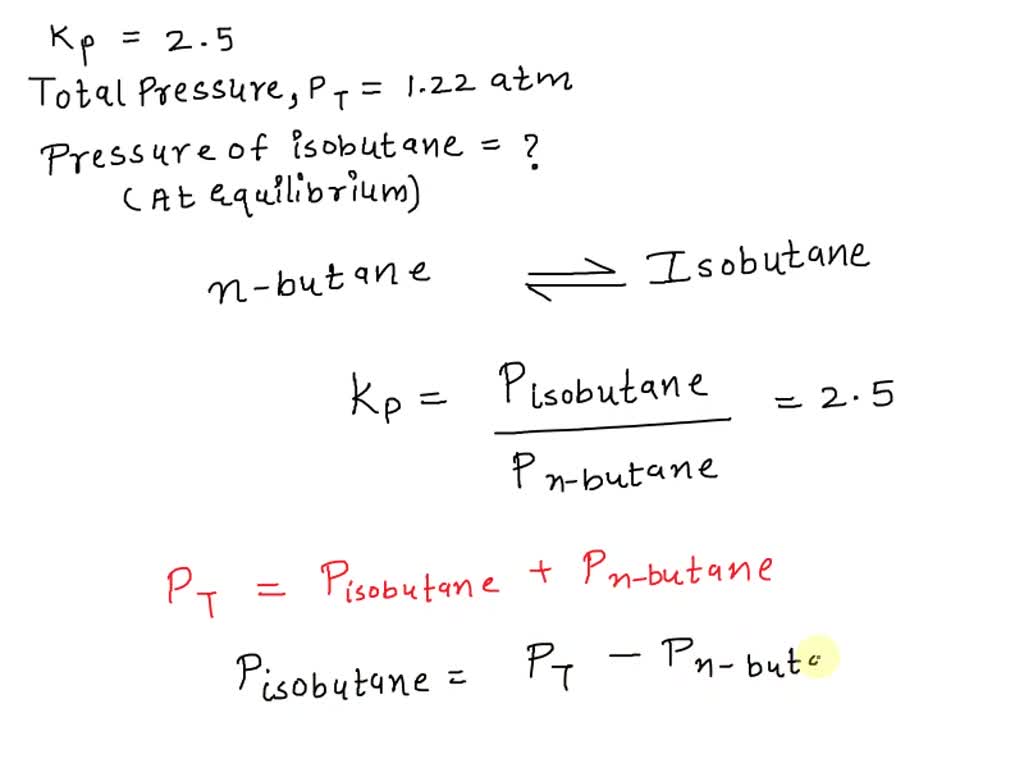
[Solved] Butane exists as two isomers, n −butane and isobutane. K P If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58g of c 4h 10208go2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. Closed oct 25, 2021 by hanitasahu. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58 gm of. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From brainly.in
Determine the number of electrons involved in bonding of isobutane If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen C 4h 10 + 213o2 → 4c o2 +5h 2o. Closed oct 25, 2021 by hanitasahu. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For 58g of c 4h 10208go2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58 gm of. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.chegg.com
Solved nButane is converted to isobutane in a continuous If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58 gm of c 4h 10 208gm o2 is. For 58g of c 4h 10208go2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. C 4h 10 + 213o2 → 4c o2 +5h 2o. Closed oct 25, 2021 by hanitasahu. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From congdongxuatnhapkhau.com
What Is The Difference Between Isobutane And NButane? If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58 gm of c 4h 10 208gm o2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58g of c 4h 10208go2 is. Closed oct 25, 2021 by hanitasahu. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.coursehero.com
[Solved] . nButane is converted to isobutane in a continuous If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen Closed oct 25, 2021 by hanitasahu. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58g of c 4h 10208go2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For 58 gm of. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.chegg.com
Solved nButane is converted to isobutane in a continuous If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58g of c 4h 10208go2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For 58 gm of c 4h 10 208gm o2 is.. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From brainly.in
Lpg is a mixture of n butane and isobutane the volume of oxygen needed If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58g of c 4h 10208go2 is. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For 58 gm of c 4h 10 208gm o2 is. Closed oct 25, 2021 by hanitasahu. C 4h 10 +. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.numerade.com
SOLVED In a sample of gaseous butane, two forms are present one is n If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58 gm of c 4h 10 208gm o2 is. For 58g of c 4h 10208go2 is. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. C 4h 10 + 213o2 → 4c o2 +5h 2o.. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.answersarena.com
[Solved] nbutane change to isobutane ( n )Butane ( lef If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen For 58 gm of c 4h 10 208gm o2 is. For 58g of c 4h 10208go2 is. C 4h 10 + 213o2 → 4c o2 +5h 2o. Closed oct 25, 2021 by hanitasahu. C 4h 10 + 213o2 → 4c o2 +5h 2o. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.
From www.toppr.com
If isobutane and n butane are present in a gas, then how much oxygen If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen C 4h 10 + 213o2 → 4c o2 +5h 2o. Closed oct 25, 2021 by hanitasahu. C 4h 10 + 213o2 → 4c o2 +5h 2o. For 58g of c 4h 10208go2 is. The correct answer is isobutane and n−butane c4h10 have same molecular formula;c4h10 + 132 o2 → 4co2+5h2o 58 g of c4h10 require→ 208. For 58 gm of. If Isobutane And N Butane Are Present In A Gas Then How Much Oxygen.