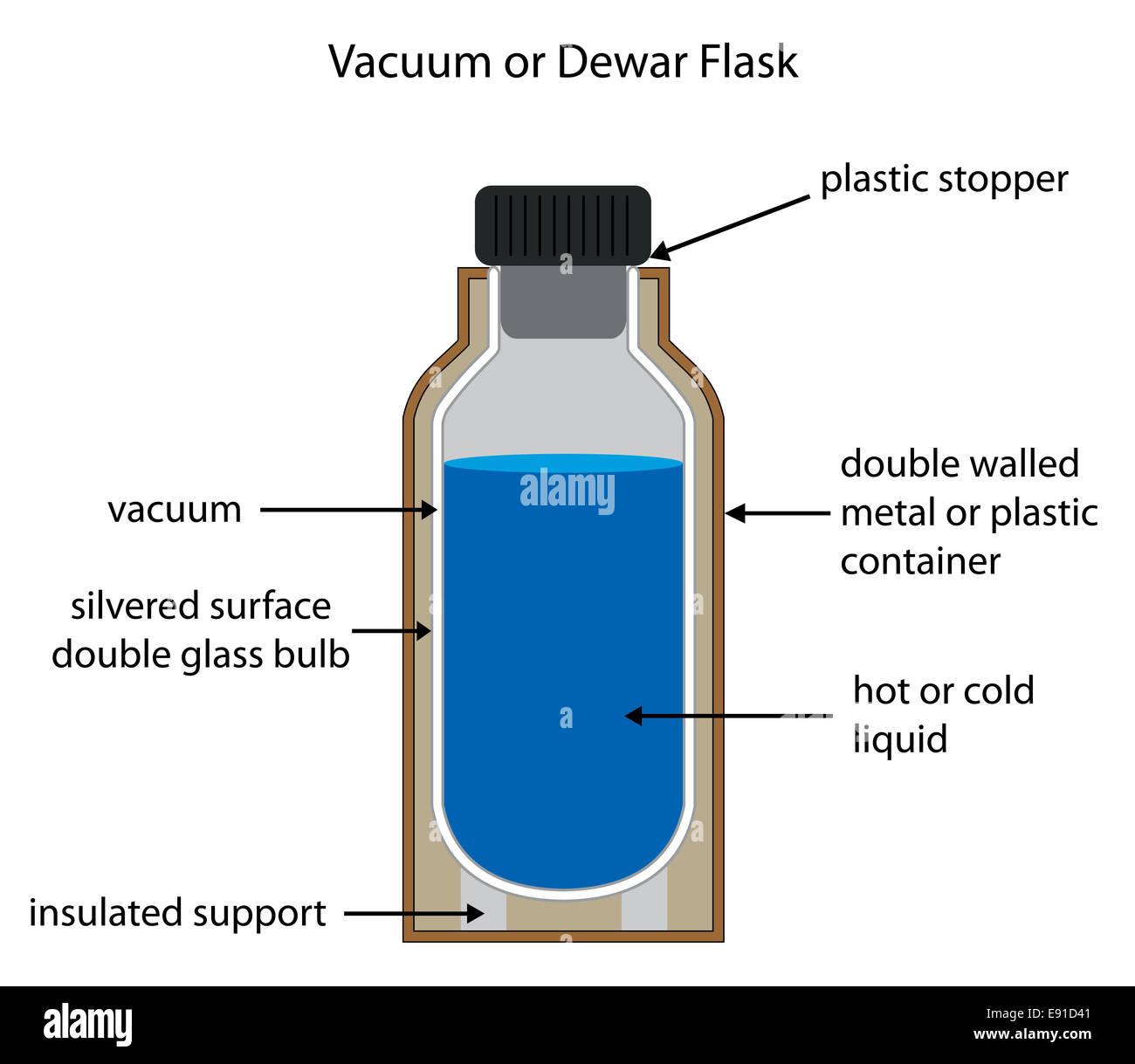
Can Liquid Exist In A Vacuum . However, there is no such a thing as perfect. Technically most materials will sublimate under vacuum. Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? Yes, liquids can exist in a high quality vacuum under certain conditions. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. In a vacuum chamber, the pressure is significantly. Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. What would happen to an ordinary glass of water in outer space? No, a liquid cannot exist in a total vacuum because it requires some amount of pressure to maintain its liquid state.
from www.alamy.com
Yes, liquids can exist in a high quality vacuum under certain conditions. What would happen to an ordinary glass of water in outer space? Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? Technically most materials will sublimate under vacuum. No, a liquid cannot exist in a total vacuum because it requires some amount of pressure to maintain its liquid state. However, there is no such a thing as perfect. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. In a vacuum chamber, the pressure is significantly.
Dewar or vacuum flask fully labeled diagram with editable layers Stock
Can Liquid Exist In A Vacuum When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. However, there is no such a thing as perfect. Technically most materials will sublimate under vacuum. Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? Yes, liquids can exist in a high quality vacuum under certain conditions. What would happen to an ordinary glass of water in outer space? Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. No, a liquid cannot exist in a total vacuum because it requires some amount of pressure to maintain its liquid state. In a vacuum chamber, the pressure is significantly.
From www.youtube.com
Liquid Ring Vacuum Pump Working Principle YouTube Can Liquid Exist In A Vacuum Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is. Can Liquid Exist In A Vacuum.
From www.kidpid.com
States of Water Kidpid Can Liquid Exist In A Vacuum When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. Technically most materials will sublimate under vacuum. Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in. Can Liquid Exist In A Vacuum.
From www.rocker.com.tw
Vacuum Filtration Setup a vacuum filtration & select right apparatus Can Liquid Exist In A Vacuum In a vacuum chamber, the pressure is significantly. Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? Technically most materials will sublimate under vacuum. No, a liquid cannot exist in a total vacuum because it requires some amount of pressure to. Can Liquid Exist In A Vacuum.
From www.fed.it
with liquid ring vacuum pump Can Liquid Exist In A Vacuum What would happen to an ordinary glass of water in outer space? When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. No, a liquid cannot exist in a total vacuum because it requires some amount of. Can Liquid Exist In A Vacuum.
From vaccent.co.za
How Nash Liquid Ring Vacuum Pumps Work, Vacuum Pump Can Liquid Exist In A Vacuum In a vacuum chamber, the pressure is significantly. Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. However, there is no such a thing as perfect. Yes, liquids can exist in a high quality vacuum under certain conditions. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic. Can Liquid Exist In A Vacuum.
From www.youtube.com
Mixing liquid chemicals with vacuum ejector by Venturi principle YouTube Can Liquid Exist In A Vacuum Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. However, there is no such a thing as perfect. What would happen to an ordinary glass of water in outer space? Technically most materials will sublimate under vacuum. In a vacuum chamber, the pressure is significantly. No, a liquid cannot exist in a total vacuum because it. Can Liquid Exist In A Vacuum.
From www.vacculex.com
What is A Liquid Ring Vacuum Pump [Benefits & Working Principle Can Liquid Exist In A Vacuum However, there is no such a thing as perfect. No, a liquid cannot exist in a total vacuum because it requires some amount of pressure to maintain its liquid state. Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? Yes, liquids. Can Liquid Exist In A Vacuum.
From www.snexplores.org
Explainer What are the different states of matter? Can Liquid Exist In A Vacuum Yes, liquids can exist in a high quality vacuum under certain conditions. In a vacuum chamber, the pressure is significantly. Technically most materials will sublimate under vacuum. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no.. Can Liquid Exist In A Vacuum.
From www.instructables.com
Vacuum Powered Liquid Extractor! 6 Steps (with Pictures) Instructables Can Liquid Exist In A Vacuum When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. However, there is no such a thing as perfect. Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. Yes, liquids can exist in. Can Liquid Exist In A Vacuum.
From mechanicalengineeringsite.com
Liquid Ring Vacuum Pump Working Principle and Pumping System Can Liquid Exist In A Vacuum Yes, liquids can exist in a high quality vacuum under certain conditions. Technically most materials will sublimate under vacuum. In a vacuum chamber, the pressure is significantly. Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? However, there is no such. Can Liquid Exist In A Vacuum.
From www.pinterest.com
What Is a Vacuum in Science? Definition and Examples Definition of Can Liquid Exist In A Vacuum Yes, liquids can exist in a high quality vacuum under certain conditions. What would happen to an ordinary glass of water in outer space? When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. Technically most materials. Can Liquid Exist In A Vacuum.
From gelcoatsystems.com
GelCoat Systems Closed Mold Vacuum Infusion Molding Can Liquid Exist In A Vacuum However, there is no such a thing as perfect. Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. Technically most materials will sublimate. Can Liquid Exist In A Vacuum.
From www.youtube.com
Liquid Ring Vacuum Pump Explained with Animation (with english Can Liquid Exist In A Vacuum Technically most materials will sublimate under vacuum. What would happen to an ordinary glass of water in outer space? However, there is no such a thing as perfect. Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? In a vacuum chamber,. Can Liquid Exist In A Vacuum.
From www.vacculex.com
Liquid Ring Vacuum Pump Working Principle How it Works Can Liquid Exist In A Vacuum However, there is no such a thing as perfect. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. In a vacuum chamber, the pressure is significantly. No, a liquid cannot exist in a total vacuum because. Can Liquid Exist In A Vacuum.
From www.bestbuy.com
Customer Reviews Shark HydroVac Cordless Pro XL 3in1 Vacuum, Mop and Can Liquid Exist In A Vacuum Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. Yes, liquids can exist in a high quality vacuum under certain conditions. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. No, a. Can Liquid Exist In A Vacuum.
From joizaxhnb.blob.core.windows.net
Hero Cannot Exist In A Vacuum at Florence Manning blog Can Liquid Exist In A Vacuum Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? What would happen to an ordinary glass of water in outer space? When you heat a liquid more molecules. Can Liquid Exist In A Vacuum.
From www.youtube.com
Chamber Vacuum Sealer How to seal liquids Breville Commercial YouTube Can Liquid Exist In A Vacuum When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. In a vacuum chamber, the pressure is significantly. Yes, liquids can exist in a high quality vacuum under certain conditions. However, there is no such a thing. Can Liquid Exist In A Vacuum.
From www.alamy.com
Dewar or vacuum flask fully labeled diagram with editable layers Stock Can Liquid Exist In A Vacuum When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. Yes, liquids can exist in a high quality vacuum under certain conditions. Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. Technically most. Can Liquid Exist In A Vacuum.
From www.pinterest.co.uk
The triple point of water is the only temperature at which water can Can Liquid Exist In A Vacuum When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. In a vacuum chamber, the pressure is significantly. Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. No, a liquid cannot exist in. Can Liquid Exist In A Vacuum.
From www.pts-thai.com
Liquid Ring Vacuum Pump PTS Process Technology Can Liquid Exist In A Vacuum In a vacuum chamber, the pressure is significantly. No, a liquid cannot exist in a total vacuum because it requires some amount of pressure to maintain its liquid state. Yes, liquids can exist in a high quality vacuum under certain conditions. However, there is no such a thing as perfect. Could you start with a frozen solid sample of a. Can Liquid Exist In A Vacuum.
From www.youtube.com
How to use Vacuum Liquid Perfume Filling Machine YouTube Can Liquid Exist In A Vacuum Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? However, there is no such a thing as perfect. No, a liquid cannot exist in a total vacuum because it requires some amount of pressure to maintain its liquid state. When you. Can Liquid Exist In A Vacuum.
From www.vacuumpumpmanufacturers.com
Liquid Ring Vacuum Pump Manufacturers Suppliers Can Liquid Exist In A Vacuum When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. What would happen to an ordinary glass of water in outer space? Could you start with a frozen solid sample of a low vapor pressure liquid, and. Can Liquid Exist In A Vacuum.
From chem.libretexts.org
D Setup of vacuum filtration Chemistry LibreTexts Can Liquid Exist In A Vacuum Technically most materials will sublimate under vacuum. No, a liquid cannot exist in a total vacuum because it requires some amount of pressure to maintain its liquid state. When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is. Can Liquid Exist In A Vacuum.
From courses.lumenlearning.com
Phase Changes Boundless Chemistry Can Liquid Exist In A Vacuum In a vacuum chamber, the pressure is significantly. Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor. Can Liquid Exist In A Vacuum.
From vacaero.com
The Fundamentals of Vacuum Theory Part 3 Can Liquid Exist In A Vacuum Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? No, a liquid cannot exist in a total vacuum because it requires some amount of pressure to maintain its liquid state. In a vacuum chamber, the pressure is significantly. What would happen. Can Liquid Exist In A Vacuum.
From www.youtube.com
Liquid Nitrogen in a Vacuum Chamber Fail TKOR Experimenting With Can Liquid Exist In A Vacuum Yes, liquids can exist in a high quality vacuum under certain conditions. Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. In a vacuum chamber, the pressure is significantly. No, a liquid cannot exist in a total vacuum because it requires some amount of pressure to maintain its liquid state. Technically most materials will sublimate under. Can Liquid Exist In A Vacuum.
From netsolwater.com
What are vacuum evaporation and it uses in treatment of wastewater Can Liquid Exist In A Vacuum When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. What would happen to an ordinary glass of water in outer space? In a vacuum chamber, the pressure is significantly. Technically most materials will sublimate under vacuum.. Can Liquid Exist In A Vacuum.
From www.vacuumpumpmanufacturers.com
Liquid Ring Vacuum Pump Manufacturers Suppliers Can Liquid Exist In A Vacuum Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? However, there is no such a thing as perfect. What would happen to an ordinary glass of water in outer space? Since perfect vacuum has no pressure, all liquids boil in a. Can Liquid Exist In A Vacuum.
From www.iqs.co.th
Vacuum System Package Can Liquid Exist In A Vacuum Technically most materials will sublimate under vacuum. Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? No, a liquid cannot exist in a total vacuum because it requires. Can Liquid Exist In A Vacuum.
From www.semanticscholar.org
Figure 1 from The Application of Vacuum Liquid Chromatography to the Can Liquid Exist In A Vacuum Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. Yes, liquids can exist in a high quality vacuum under certain conditions. No, a liquid cannot exist in a total vacuum because it requires some amount of pressure to maintain its liquid state. Could you start with a frozen solid sample of a low vapor pressure liquid,. Can Liquid Exist In A Vacuum.
From mechanicalengineeringsite.com
Liquid Ring Vacuum Pump Working Principle and Pumping System Can Liquid Exist In A Vacuum Technically most materials will sublimate under vacuum. Since perfect vacuum has no pressure, all liquids boil in a perfect vacuum. What would happen to an ordinary glass of water in outer space? When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid. Can Liquid Exist In A Vacuum.
From owlcation.com
All About Matter An Introduction to the Basics Owlcation Can Liquid Exist In A Vacuum No, a liquid cannot exist in a total vacuum because it requires some amount of pressure to maintain its liquid state. Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? When you heat a liquid more molecules will leave the liquid. Can Liquid Exist In A Vacuum.
From byjus.com
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law Can Liquid Exist In A Vacuum Technically most materials will sublimate under vacuum. Yes, liquids can exist in a high quality vacuum under certain conditions. What would happen to an ordinary glass of water in outer space? When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and. Can Liquid Exist In A Vacuum.
From www.numerade.com
SOLVEDCan an electric field exist in a vacuum? Explain. Can Liquid Exist In A Vacuum Yes, liquids can exist in a high quality vacuum under certain conditions. Could you start with a frozen solid sample of a low vapor pressure liquid, and then heat it up in a vacuum until it melts into a liquid? Technically most materials will sublimate under vacuum. No, a liquid cannot exist in a total vacuum because it requires some. Can Liquid Exist In A Vacuum.
From www.chegg.com
Solved No liquid can exist as liquid at vacuum 273 °K А О Can Liquid Exist In A Vacuum However, there is no such a thing as perfect. What would happen to an ordinary glass of water in outer space? When you heat a liquid more molecules will leave the liquid into the vapor (due to higher kinetic energies) than molecules from the vapor phase into the liquid and the interface is no. Technically most materials will sublimate under. Can Liquid Exist In A Vacuum.