Combustion Definition Ks3 . When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. Methane + oxygen → carbon dioxide + water. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. In a complete combustion reaction, a fuel reacts with oxygen to produce carbon dioxide and water. Combustion is another name for burning. I can describe oxidation reactions, including combustion and rusting, and name the products of some oxidation reactions. , or burning, is an example of an oxidation reaction. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. When a substance reacts to combine with oxygen, this. Combustion requires fuel, heat and oxygen, and will not happen without one of them. Combustion is a fancy name for burning. This is because a fuel reacts with oxygen to release energy.
from www.teachoo.com
In a combustion reaction, fuel is burned and reacts with oxygen to release energy. Combustion is another name for burning. Methane + oxygen → carbon dioxide + water. This is because a fuel reacts with oxygen to release energy. When a substance reacts to combine with oxygen, this. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. Combustion is a fancy name for burning. Combustion requires fuel, heat and oxygen, and will not happen without one of them. In a complete combustion reaction, a fuel reacts with oxygen to produce carbon dioxide and water.
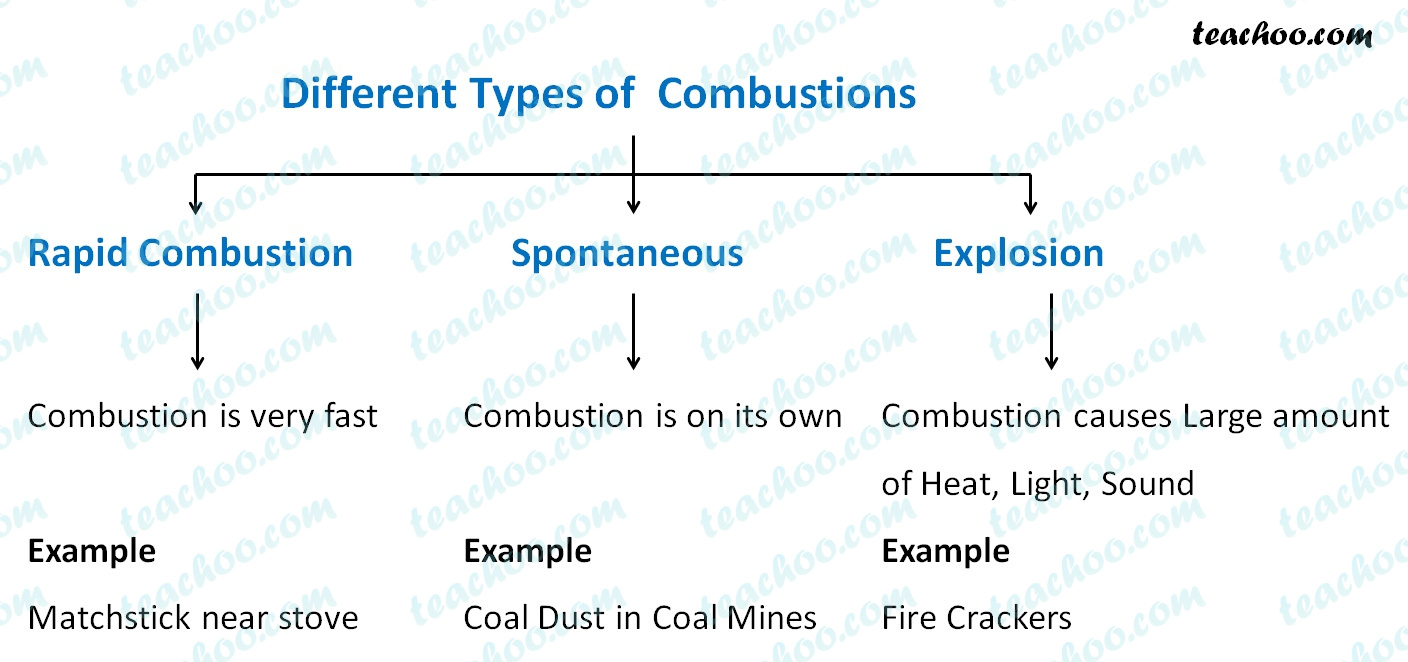
Different Types of Combustion with Examples Teachoo Concepts
Combustion Definition Ks3 When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. , or burning, is an example of an oxidation reaction. Combustion is another name for burning. When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. I can describe oxidation reactions, including combustion and rusting, and name the products of some oxidation reactions. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. This is because a fuel reacts with oxygen to release energy. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. In a complete combustion reaction, a fuel reacts with oxygen to produce carbon dioxide and water. When a substance reacts to combine with oxygen, this. Combustion is a fancy name for burning. Combustion requires fuel, heat and oxygen, and will not happen without one of them. Methane + oxygen → carbon dioxide + water.
From www.tes.com
Combustion KS3 Teaching Resources Combustion Definition Ks3 I can describe oxidation reactions, including combustion and rusting, and name the products of some oxidation reactions. Combustion is another name for burning. Methane + oxygen → carbon dioxide + water. Combustion requires fuel, heat and oxygen, and will not happen without one of them. Combustion is a fancy name for burning. When a substance reacts to combine with oxygen,. Combustion Definition Ks3.
From www.tes.com
Combustion Burning Fuels Year 8 Lesson PowerPoint (KS3 8Ea Combustion Definition Ks3 Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. Combustion is another name for burning. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion requires fuel, heat and oxygen, and will not happen without one of them. When you. Combustion Definition Ks3.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID506027 Combustion Definition Ks3 , or burning, is an example of an oxidation reaction. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. I can describe oxidation reactions, including combustion and rusting, and name the products of some oxidation reactions. In a combustion reaction, fuel is burned and reacts with oxygen. Combustion Definition Ks3.
From www.youtube.com
Definition of Combustion for class 8 science. YouTube Combustion Definition Ks3 I can describe oxidation reactions, including combustion and rusting, and name the products of some oxidation reactions. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. When a substance reacts to combine with oxygen, this. , or burning, is an example of an oxidation reaction. Methane +. Combustion Definition Ks3.
From www.abcworksheet.com
What is Combustion Definition of Combustion Combustion Definition Ks3 Methane + oxygen → carbon dioxide + water. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. This is because a fuel reacts with oxygen to release energy. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. In a combustion. Combustion Definition Ks3.
From slideshare.net
form1sciencechapter5part2 Combustion Definition Ks3 Methane + oxygen → carbon dioxide + water. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. Combustion requires fuel, heat and oxygen, and will not happen without one of them. Combustion is another name for burning. In a combustion. Combustion Definition Ks3.
From giotlkfth.blob.core.windows.net
Combustion Definition Ks3 at Phillip Fry blog Combustion Definition Ks3 Combustion requires fuel, heat and oxygen, and will not happen without one of them. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. When you burn a hydrocarbon, you can have. Combustion Definition Ks3.
From www.javatpoint.com
Combustion Definition JavaTpoint Combustion Definition Ks3 , or burning, is an example of an oxidation reaction. Combustion requires fuel, heat and oxygen, and will not happen without one of them. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion is a fancy name for burning. When you burn a hydrocarbon, you can have complete combustion or incomplete combustion.. Combustion Definition Ks3.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID506027 Combustion Definition Ks3 When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. In a complete combustion reaction, a fuel reacts with oxygen to produce carbon dioxide and water. This is because a fuel reacts with oxygen to release energy. Methane + oxygen →. Combustion Definition Ks3.
From www.shalom-education.com
Fuels and Combustion KS3 Chemistry Revision Combustion Definition Ks3 In a complete combustion reaction, a fuel reacts with oxygen to produce carbon dioxide and water. Combustion is a fancy name for burning. Combustion requires fuel, heat and oxygen, and will not happen without one of them. This is because a fuel reacts with oxygen to release energy. Combustion requires fuel, oxygen, and an initial source of energy, usually heat,. Combustion Definition Ks3.
From giotlkfth.blob.core.windows.net
Combustion Definition Ks3 at Phillip Fry blog Combustion Definition Ks3 This is because a fuel reacts with oxygen to release energy. Combustion requires fuel, heat and oxygen, and will not happen without one of them. Methane + oxygen → carbon dioxide + water. When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. When a substance reacts to combine with oxygen, this. Combustion is a fancy name. Combustion Definition Ks3.
From giotlkfth.blob.core.windows.net
Combustion Definition Ks3 at Phillip Fry blog Combustion Definition Ks3 This is because a fuel reacts with oxygen to release energy. Combustion is another name for burning. In a complete combustion reaction, a fuel reacts with oxygen to produce carbon dioxide and water. Combustion is a fancy name for burning. When a substance reacts to combine with oxygen, this. Combustion requires fuel, oxygen, and an initial source of energy, usually. Combustion Definition Ks3.
From www.teachoo.com
Different Types of Combustion with Examples Teachoo Concepts Combustion Definition Ks3 This is because a fuel reacts with oxygen to release energy. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. Combustion is another name for burning. , or burning, is an example of an. Combustion Definition Ks3.
From www.tes.com
Burning fuels KS3 Activate Science Teaching Resources Combustion Definition Ks3 I can describe oxidation reactions, including combustion and rusting, and name the products of some oxidation reactions. In a complete combustion reaction, a fuel reacts with oxygen to produce carbon dioxide and water. Methane + oxygen → carbon dioxide + water. When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. Combustion requires fuel, heat and oxygen,. Combustion Definition Ks3.
From www.tes.com
KS3 Chemistry AQA C2 2 5 Exploring Combustion Teaching Resources Combustion Definition Ks3 When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. Combustion is a fancy name for burning. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. I can describe oxidation reactions, including combustion and rusting, and name the products of some oxidation reactions. Combustion requires fuel, oxygen, and an initial source of. Combustion Definition Ks3.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation ID6818296 Combustion Definition Ks3 , or burning, is an example of an oxidation reaction. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. When a substance reacts to combine with oxygen, this. When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. Combustion requires fuel, heat and oxygen,. Combustion Definition Ks3.
From www.tes.com
KS3 Science / Chemistry Combustion worksheet Teaching Resources Combustion Definition Ks3 Combustion is a fancy name for burning. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. In a complete combustion reaction, a fuel reacts with oxygen to produce carbon dioxide and water. I can describe oxidation reactions, including combustion and rusting, and name the products of some oxidation reactions. , or burning, is an example. Combustion Definition Ks3.
From www.tes.com
Combustion KS3 Activate Science Teaching Resources Combustion Definition Ks3 Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion is a fancy name for burning. I can describe oxidation reactions, including combustion and rusting, and name the products of some. Combustion Definition Ks3.
From www.tes.com
Oxidation (of Fuels) Year 8 Lesson PowerPoint (KS3 8Eb) Combustion Combustion Definition Ks3 Combustion is a fancy name for burning. In a complete combustion reaction, a fuel reacts with oxygen to produce carbon dioxide and water. When a substance reacts to combine with oxygen, this. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. When you burn a hydrocarbon, you can have complete combustion or incomplete. Combustion Definition Ks3.
From www.tes.com
Secondary chemistry teaching resources Chemical reactions TES Combustion Definition Ks3 In a combustion reaction, fuel is burned and reacts with oxygen to release energy. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion is another name for burning. Combustion requires fuel, heat and oxygen, and will not happen without one of them. When you burn a hydrocarbon, you can have complete combustion. Combustion Definition Ks3.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID6818296 Combustion Definition Ks3 Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. I can describe oxidation reactions, including combustion and rusting, and name. Combustion Definition Ks3.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID506027 Combustion Definition Ks3 This is because a fuel reacts with oxygen to release energy. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. When a substance reacts to combine with oxygen, this. Methane + oxygen → carbon dioxide + water. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in. Combustion Definition Ks3.
From giotlkfth.blob.core.windows.net
Combustion Definition Ks3 at Phillip Fry blog Combustion Definition Ks3 Combustion is another name for burning. , or burning, is an example of an oxidation reaction. This is because a fuel reacts with oxygen to release energy. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Methane + oxygen. Combustion Definition Ks3.
From giotlkfth.blob.core.windows.net
Combustion Definition Ks3 at Phillip Fry blog Combustion Definition Ks3 This is because a fuel reacts with oxygen to release energy. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. I can describe oxidation reactions, including combustion and rusting, and name the products of. Combustion Definition Ks3.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Combustion Definition Ks3 When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. Combustion is another name for burning. I can describe oxidation reactions, including combustion and rusting, and name the products of some oxidation reactions. This is because a fuel reacts with oxygen to release energy. When a substance reacts to combine with oxygen, this. In a complete combustion. Combustion Definition Ks3.
From www.youtube.com
KS3 Combustion, Fossil Fuels and Pollution 1 Products of Combustion Combustion Definition Ks3 I can describe oxidation reactions, including combustion and rusting, and name the products of some oxidation reactions. , or burning, is an example of an oxidation reaction. Combustion requires fuel, heat and oxygen, and will not happen without one of them. Combustion is a fancy name for burning. Combustion requires fuel, oxygen, and an initial source of energy, usually heat,. Combustion Definition Ks3.
From exoknraag.blob.core.windows.net
Combustion Reaction Definition Science at Tina Pritchard blog Combustion Definition Ks3 When a substance reacts to combine with oxygen, this. When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. , or burning, is an example of an oxidation reaction. This is because a fuel reacts. Combustion Definition Ks3.
From www.teacherspayteachers.com
Combustion (KS3) by CMGs Science lessons TPT Combustion Definition Ks3 Combustion is a fancy name for burning. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. Methane + oxygen → carbon dioxide + water. When a substance reacts to combine with oxygen, this. In a complete combustion reaction, a fuel reacts with oxygen to produce carbon dioxide. Combustion Definition Ks3.
From www.tes.com
Combustion KS3 Activate Science Teaching Resources Combustion Definition Ks3 Combustion is a fancy name for burning. Methane + oxygen → carbon dioxide + water. , or burning, is an example of an oxidation reaction. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. This is because a fuel reacts with oxygen to release energy. In a complete combustion reaction, a fuel reacts. Combustion Definition Ks3.
From www.tes.com
Combustion Year 8 Topic 5 full lessons (KS3 8E) by OnSpecScience Combustion Definition Ks3 Methane + oxygen → carbon dioxide + water. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. When a substance reacts to combine with oxygen, this. Combustion is another name for burning. I can. Combustion Definition Ks3.
From gamesmartz.com
Combustion Definition & Image GameSmartz Combustion Definition Ks3 Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3 chemistry guide. Combustion is a fancy name for burning. , or burning, is an example of an oxidation reaction. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. Combustion is another name for burning. When. Combustion Definition Ks3.
From www.chemicals.co.uk
What is Combustion in Chemistry? The Chemistry Blog Combustion Definition Ks3 Combustion is another name for burning. , or burning, is an example of an oxidation reaction. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. Combustion requires fuel, heat and oxygen, and will not happen without one of them. This is because a fuel reacts with oxygen to release energy. When you burn. Combustion Definition Ks3.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation ID6818296 Combustion Definition Ks3 This is because a fuel reacts with oxygen to release energy. When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. Combustion requires fuel, oxygen, and an initial source of energy, usually heat, to start the reaction. In a complete combustion reaction, a fuel reacts with oxygen to produce carbon dioxide and water. In a combustion reaction,. Combustion Definition Ks3.
From www.tes.com
Combustion Teaching Resources Combustion Definition Ks3 Combustion requires fuel, heat and oxygen, and will not happen without one of them. In a combustion reaction, fuel is burned and reacts with oxygen to release energy. Combustion is a fancy name for burning. When you burn a hydrocarbon, you can have complete combustion or incomplete combustion. , or burning, is an example of an oxidation reaction. I can. Combustion Definition Ks3.
From www.tes.com
Burning fuels and Combustion KS3 Science Teaching Resources Combustion Definition Ks3 When a substance reacts to combine with oxygen, this. In a complete combustion reaction, a fuel reacts with oxygen to produce carbon dioxide and water. Methane + oxygen → carbon dioxide + water. Combustion is a fancy name for burning. Learn the definition of combustion, the equation for combustion and what a combustion reaction is in this bbc bitesize ks3. Combustion Definition Ks3.