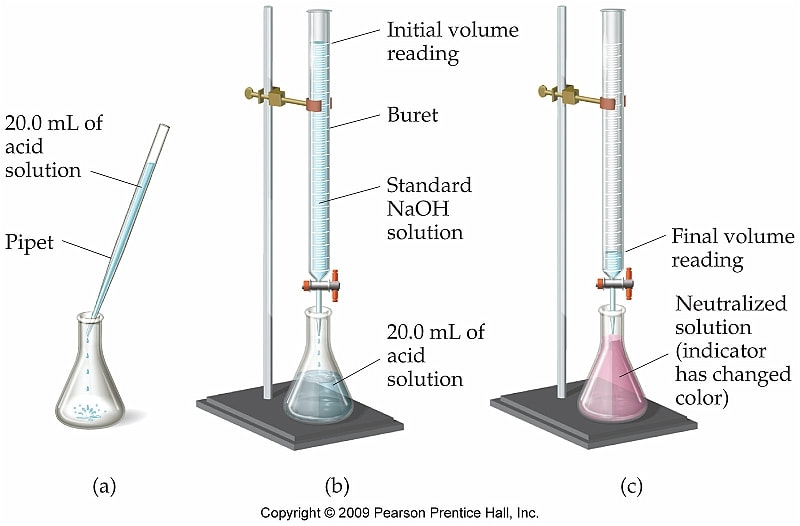
Diagram For Titration . In an indicator based titration you add another. It is based on the neutralization reaction, where. There are two basic types of acid base titrations, indicator and potentiometric. This required practical involves using. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. This is an outline method for carrying out a titration in which an acid is added to alkali. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Use the pipette and pipette filler to add 25 cm 3 of. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid.
from giofldval.blob.core.windows.net
It is based on the neutralization reaction, where. Use the pipette and pipette filler to add 25 cm 3 of. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. This is an outline method for carrying out a titration in which an acid is added to alkali. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. This required practical involves using. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid.
Diagram For Titration at Leroy Perez blog
Diagram For Titration Use the pipette and pipette filler to add 25 cm 3 of. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. There are two basic types of acid base titrations, indicator and potentiometric. Use the pipette and pipette filler to add 25 cm 3 of. It is based on the neutralization reaction, where. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. This required practical involves using. This is an outline method for carrying out a titration in which an acid is added to alkali. In an indicator based titration you add another.
From www.microlit.com
An Advanced Guide to Titration Microlit Diagram For Titration Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Use the pipette and pipette filler to add 25 cm 3 of. There are two basic types. Diagram For Titration.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Diagram For Titration Use the pipette and pipette filler to add 25 cm 3 of. This is an outline method for carrying out a titration in which an acid is added to alkali. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. Sketch out a plot representing the titration of a weak. Diagram For Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Diagram For Titration There are two basic types of acid base titrations, indicator and potentiometric. It is based on the neutralization reaction, where. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. This required practical involves using. Learn about the titration technique through a practical experiment. Diagram For Titration.
From mavink.com
Titration Diagram Labeled Diagram For Titration This required practical involves using. Use the pipette and pipette filler to add 25 cm 3 of. This is an outline method for carrying out a titration in which an acid is added to alkali. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. In an indicator based titration. Diagram For Titration.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Diagram For Titration This is an outline method for carrying out a titration in which an acid is added to alkali. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration. Diagram For Titration.
From mavink.com
Titration Diagram Diagram For Titration In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. This required practical. Diagram For Titration.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Diagram For Titration This required practical involves using. This is an outline method for carrying out a titration in which an acid is added to alkali. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In an indicator based titration you add another. Learn about the. Diagram For Titration.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources Diagram For Titration Use the pipette and pipette filler to add 25 cm 3 of. This is an outline method for carrying out a titration in which an acid is added to alkali. In an indicator based titration you add another. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. It is. Diagram For Titration.
From www.researchgate.net
Complexometric (FeEDTA) titration for determination of iron in water Diagram For Titration This required practical involves using. There are two basic types of acid base titrations, indicator and potentiometric. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. This is an outline method for carrying out a titration in which an acid is added to alkali. It is based on the neutralization reaction, where.. Diagram For Titration.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram For Titration There are two basic types of acid base titrations, indicator and potentiometric. This is an outline method for carrying out a titration in which an acid is added to alkali. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. Use the pipette and pipette filler to add 25 cm. Diagram For Titration.
From www.youtube.com
Titration tube How to draw well labelled Titration Tube Titration Diagram For Titration This is an outline method for carrying out a titration in which an acid is added to alkali. This required practical involves using. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or. Diagram For Titration.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Diagram For Titration There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. Use the pipette and pipette filler to add 25 cm 3 of. This required practical involves using. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. Diagram For Titration.
From www.savemyexams.co.uk
Titrations (1.2.9) DP IB Chemistry SL Revision Notes 2016 Save My Diagram For Titration It is based on the neutralization reaction, where. Use the pipette and pipette filler to add 25 cm 3 of. In an indicator based titration you add another. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. Determination of the reacting volumes of solutions of a strong acid and. Diagram For Titration.
From giofldval.blob.core.windows.net
Diagram For Titration at Leroy Perez blog Diagram For Titration There are two basic types of acid base titrations, indicator and potentiometric. Use the pipette and pipette filler to add 25 cm 3 of. In an indicator based titration you add another. It is based on the neutralization reaction, where. This required practical involves using. Learn about the titration technique through a practical experiment to determine the reacting volumes and. Diagram For Titration.
From www.researchgate.net
Basic process flow for the automated titration. Download Scientific Diagram For Titration It is based on the neutralization reaction, where. There are two basic types of acid base titrations, indicator and potentiometric. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. In an indicator based titration you add another. Sketch out a plot representing the titration of a weak monoprotic acid by a strong. Diagram For Titration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Diagram For Titration It is based on the neutralization reaction, where. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Learn about the titration technique through. Diagram For Titration.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Diagram For Titration There are two basic types of acid base titrations, indicator and potentiometric. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Use the pipette and pipette filler to add 25 cm 3 of. This required practical involves using. Learn about the titration technique. Diagram For Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Diagram For Titration There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. This is an outline method for carrying out a titration in which an acid is added to alkali. It is based on the. Diagram For Titration.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali Diagram For Titration It is based on the neutralization reaction, where. There are two basic types of acid base titrations, indicator and potentiometric. This required practical involves using. In an indicator based titration you add another. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. Sketch out a plot representing the titration. Diagram For Titration.
From ck12.org
Titration CK12 Foundation Diagram For Titration There are two basic types of acid base titrations, indicator and potentiometric. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. This required practical involves using. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration.. Diagram For Titration.
From giofldval.blob.core.windows.net
Diagram For Titration at Leroy Perez blog Diagram For Titration This required practical involves using. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. There are two basic types of acid base titrations,. Diagram For Titration.
From sachiacidbase.weebly.com
Titrations Sachi's Acids and Bases Diagram For Titration In an indicator based titration you add another. It is based on the neutralization reaction, where. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. This is an outline method for carrying out a titration in which an acid is added to alkali. Use the pipette and pipette filler. Diagram For Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Diagram For Titration It is based on the neutralization reaction, where. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. In an indicator based titration you add another. Use the pipette and pipette filler to add 25 cm 3 of. Sketch out a plot representing the titration of a weak monoprotic acid by a strong. Diagram For Titration.
From mungfali.com
Titration Process Diagram For Titration This is an outline method for carrying out a titration in which an acid is added to alkali. In an indicator based titration you add another. Use the pipette and pipette filler to add 25 cm 3 of. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. Sketch out. Diagram For Titration.
From giofldval.blob.core.windows.net
Diagram For Titration at Leroy Perez blog Diagram For Titration This required practical involves using. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. It is based on the neutralization reaction, where. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Sketch out a plot representing the titration of a weak. Diagram For Titration.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Diagram For Titration This is an outline method for carrying out a titration in which an acid is added to alkali. Use the pipette and pipette filler to add 25 cm 3 of. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. This required practical involves using. Sketch out a plot representing the titration of. Diagram For Titration.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Diagram For Titration Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. There are two basic types of acid base titrations, indicator and potentiometric. Use the pipette and pipette filler to add 25 cm 3. Diagram For Titration.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Diagram For Titration There are two basic types of acid base titrations, indicator and potentiometric. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. This required practical involves using. Use the pipette and pipette filler. Diagram For Titration.
From stock.adobe.com
Acid base titration experiment and phases of color change during Diagram For Titration Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. This required practical involves using. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. There are two basic types of acid base titrations, indicator and potentiometric. Sketch out a plot representing the. Diagram For Titration.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Diagram For Titration This is an outline method for carrying out a titration in which an acid is added to alkali. In an indicator based titration you add another. Use the pipette and pipette filler to add 25 cm 3 of. There are two basic types of acid base titrations, indicator and potentiometric. Sketch out a plot representing the titration of a weak. Diagram For Titration.
From www.sliderbase.com
Titration Diagram For Titration This required practical involves using. In an indicator based titration you add another. It is based on the neutralization reaction, where. This is an outline method for carrying out a titration in which an acid is added to alkali. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. There are two basic. Diagram For Titration.
From letitsnowglobe.co.uk
Titration procedure pdf Diagram For Titration There are two basic types of acid base titrations, indicator and potentiometric. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid. It is based on the neutralization reaction, where. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak. Diagram For Titration.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Diagram For Titration This required practical involves using. There are two basic types of acid base titrations, indicator and potentiometric. This is an outline method for carrying out a titration in which an acid is added to alkali. It is based on the neutralization reaction, where. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration.. Diagram For Titration.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Diagram For Titration It is based on the neutralization reaction, where. There are two basic types of acid base titrations, indicator and potentiometric. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. This required practical involves using. This is an outline method for carrying out a. Diagram For Titration.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Diagram For Titration This is an outline method for carrying out a titration in which an acid is added to alkali. This required practical involves using. Use the pipette and pipette filler to add 25 cm 3 of. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong. Diagram For Titration.