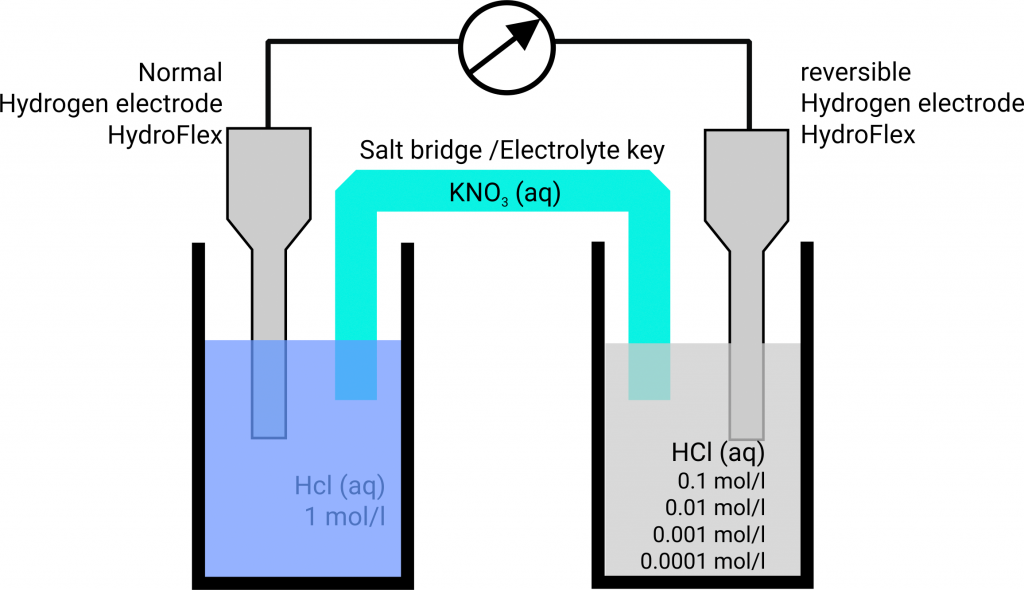
Hydrogen Electrode Acid . The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard hydrogen electrode (she). If hydrogen gas is adsorbed at 1 atm. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. An introduction to redox equilibria and electrode potentials. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The structure of the standard hydrogen electrode is. This page explains the background to standard electrode potentials.
from gaskatel.de
An introduction to redox equilibria and electrode potentials. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. This page explains the background to standard electrode potentials. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard hydrogen electrode (she). The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. The structure of the standard hydrogen electrode is. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. If hydrogen gas is adsorbed at 1 atm.
Principles of the pHvalue measurement with hydrogen electrodes Gaskatel
Hydrogen Electrode Acid The structure of the standard hydrogen electrode is. This page explains the background to standard electrode potentials. The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. The structure of the standard hydrogen electrode is. If hydrogen gas is adsorbed at 1 atm. The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard hydrogen electrode (she). The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. An introduction to redox equilibria and electrode potentials. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials.
From avopix.com
Standard hydrogen electrode diagram. Scientific Royalty Free Stock Hydrogen Electrode Acid If hydrogen gas is adsorbed at 1 atm. The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard hydrogen electrode (she). This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The choice that has been adopted is the standard hydrogen electrode, often abbreviated the. Hydrogen Electrode Acid.
From joel-chapter.blogspot.com
A Cell Contains Two Hydrogen Electrodes 35+ Pages Summary [2.8mb Hydrogen Electrode Acid An introduction to redox equilibria and electrode potentials. The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. This page explains the background to standard electrode potentials. Graphical representation of the onset. Hydrogen Electrode Acid.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Hydrogen Electrode Acid The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. This page explains the background to standard electrode potentials. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. The choice that has been adopted is the standard hydrogen electrode, often abbreviated. Hydrogen Electrode Acid.
From www.denora.com
Anodes and Cathodes for Fuel Cells De Nora Electrode and Water Hydrogen Electrode Acid The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. The structure of the standard hydrogen electrode is. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. This page explains the background to standard electrode potentials. This is a reference electrode to which all electrodes are. Hydrogen Electrode Acid.
From www.researchgate.net
Hydrogen evolution mechanism under acidic (bottom) and alkaline (top Hydrogen Electrode Acid An introduction to redox equilibria and electrode potentials. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. This page explains the background to standard electrode potentials. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The structure of the standard hydrogen electrode is. Graphical. Hydrogen Electrode Acid.
From saylordotorg.github.io
Electrochemistry Hydrogen Electrode Acid This page explains the background to standard electrode potentials. The structure of the standard hydrogen electrode is. An introduction to redox equilibria and electrode potentials. The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible. Hydrogen Electrode Acid.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Hydrogen Electrode Acid This page explains the background to standard electrode potentials. An introduction to redox equilibria and electrode potentials. If hydrogen gas is adsorbed at 1 atm. The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e.. Hydrogen Electrode Acid.
From www.slideserve.com
PPT BASIC ELECTROCHEMISTRY PowerPoint Presentation ID594481 Hydrogen Electrode Acid Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. This page explains the background to standard electrode potentials. The structure of the standard hydrogen electrode is. The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. The she consists of 1 atm of. Hydrogen Electrode Acid.
From www.youtube.com
Electrochemistry 06 Standard hydrogen electrode YouTube Hydrogen Electrode Acid This page explains the background to standard electrode potentials. The structure of the standard hydrogen electrode is. An introduction to redox equilibria and electrode potentials. The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard hydrogen electrode (she). The standard hydrogen electrode is the standard measurement of electrode potential for the. Hydrogen Electrode Acid.
From www.lambdasystem.pl
Hydrogen reference electrode Electrodes ELECTROCHEMISTRY Hydrogen Electrode Acid The structure of the standard hydrogen electrode is. The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard hydrogen electrode (she). This page explains the background to standard electrode potentials. If hydrogen gas is adsorbed at 1 atm. The standard hydrogen electrode is the standard measurement of electrode potential for the. Hydrogen Electrode Acid.
From www.youtube.com
Standard Hydrogen Electrode (SHE)/ Primary reference electrode Gas Hydrogen Electrode Acid This page explains the background to standard electrode potentials. The structure of the standard hydrogen electrode is. The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. If hydrogen gas is adsorbed at 1 atm. The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard hydrogen. Hydrogen Electrode Acid.
From www.vedantu.com
Define the reference electrode. Hydrogen Electrode Acid If hydrogen gas is adsorbed at 1 atm. An introduction to redox equilibria and electrode potentials. The structure of the standard hydrogen electrode is. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard hydrogen. Hydrogen Electrode Acid.
From brainly.in
Standard hydrogen electrode explain Brainly.in Hydrogen Electrode Acid An introduction to redox equilibria and electrode potentials. The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. The structure of the standard hydrogen electrode is. This page explains the background to standard electrode potentials. The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e.. Hydrogen Electrode Acid.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID752723 Hydrogen Electrode Acid The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard hydrogen electrode (she). This is a reference electrode to which all electrodes are calculated in terms of electrode potential. This page explains the background to standard electrode potentials. Graphical representation of the onset potentials of the her/hor and oer/orr measured using. Hydrogen Electrode Acid.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Hydrogen Electrode Acid This page explains the background to standard electrode potentials. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. An introduction to redox equilibria and electrode potentials. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The she consists of 1 atm. Hydrogen Electrode Acid.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Hydrogen Electrode Acid The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. The structure of the standard hydrogen electrode is. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. This page explains the background to standard electrode potentials. The electrode chosen as the. Hydrogen Electrode Acid.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Hydrogen Electrode Acid The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. The structure of the standard hydrogen electrode is. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. If hydrogen gas. Hydrogen Electrode Acid.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Hydrogen Electrode Acid The structure of the standard hydrogen electrode is. The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. This page explains the background to standard electrode potentials. An introduction to redox equilibria and electrode potentials. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. The she. Hydrogen Electrode Acid.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Hydrogen Electrode Acid If hydrogen gas is adsorbed at 1 atm. The structure of the standard hydrogen electrode is. The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard hydrogen electrode (she). The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. The she consists of 1 atm of. Hydrogen Electrode Acid.
From byjus.com
What is standard hydrogen electrode? Hydrogen Electrode Acid The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard. Hydrogen Electrode Acid.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that Hydrogen Electrode Acid The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. If hydrogen gas is adsorbed at 1 atm. The structure of the standard hydrogen electrode is. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The choice that. Hydrogen Electrode Acid.
From question.pandai.org
Standard Electrode Potential Hydrogen Electrode Acid The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. The structure of the standard hydrogen electrode is. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. If hydrogen gas is adsorbed at 1 atm. The electrode chosen as the zero voltage (standard. Hydrogen Electrode Acid.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Hydrogen Electrode Acid If hydrogen gas is adsorbed at 1 atm. The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard hydrogen electrode (she). The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. The choice that has been adopted is the standard hydrogen electrode, often. Hydrogen Electrode Acid.
From scienceinfo.com
What is Standard Hydrogen Electrode? Hydrogen Electrode Acid The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. This page explains the background to standard electrode potentials. The structure of the standard hydrogen electrode is. The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. If hydrogen gas is adsorbed. Hydrogen Electrode Acid.
From www.savemyexams.com
Standard Electrode Potential Edexcel International A Level Chemistry Hydrogen Electrode Acid This is a reference electrode to which all electrodes are calculated in terms of electrode potential. If hydrogen gas is adsorbed at 1 atm. The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard hydrogen electrode (she). This page explains the background to standard electrode potentials. Graphical representation of the onset. Hydrogen Electrode Acid.
From www.numerade.com
SOLVED (a) Write the halfreaction that occurs at a hydrogen electrode Hydrogen Electrode Acid This is a reference electrode to which all electrodes are calculated in terms of electrode potential. This page explains the background to standard electrode potentials. The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e.. Hydrogen Electrode Acid.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Hydrogen Electrode Acid Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. If hydrogen gas is adsorbed at 1 atm. This page explains the background to standard electrode potentials. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. An introduction to redox. Hydrogen Electrode Acid.
From monomole.com
Standard hydrogen electrode Mono Mole Hydrogen Electrode Acid This is a reference electrode to which all electrodes are calculated in terms of electrode potential. If hydrogen gas is adsorbed at 1 atm. The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. This page explains the background to standard electrode potentials. An introduction to redox equilibria and electrode. Hydrogen Electrode Acid.
From www.youtube.com
Determination of pH ( by coupling hydrogen, glass & quinhydrone Hydrogen Electrode Acid The structure of the standard hydrogen electrode is. An introduction to redox equilibria and electrode potentials. The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. If hydrogen gas is adsorbed at 1 atm. The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard hydrogen electrode. Hydrogen Electrode Acid.
From psu.pb.unizin.org
17.3 Standard Reduction Potentials Chemistry 112 Chapters 1217 of Hydrogen Electrode Acid The structure of the standard hydrogen electrode is. The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. This page explains the background to standard electrode potentials. The she consists of 1 atm of. Hydrogen Electrode Acid.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Hydrogen Electrode Acid An introduction to redox equilibria and electrode potentials. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. If hydrogen gas is adsorbed at 1 atm. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The standard hydrogen electrode is the standard. Hydrogen Electrode Acid.
From saylordotorg.github.io
Electrochemistry Hydrogen Electrode Acid If hydrogen gas is adsorbed at 1 atm. The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. Graphical representation of the onset potentials of the her/hor and oer/orr measured using a standard hydrogen electrode (she), a reversible hydrogen. The electrode chosen as the zero voltage (standard electrode) is shown. Hydrogen Electrode Acid.
From gaskatel.de
Principles of the pHvalue measurement with hydrogen electrodes Gaskatel Hydrogen Electrode Acid An introduction to redox equilibria and electrode potentials. The structure of the standard hydrogen electrode is. The she consists of 1 atm of hydrogen gas bubbled through a 1 m hcl solution, usually at room temperature. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. Graphical representation of the onset potentials of the. Hydrogen Electrode Acid.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Hydrogen Electrode Acid The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. If hydrogen gas is adsorbed at 1 atm. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. An introduction to. Hydrogen Electrode Acid.
From www.youtube.com
Standard Hydrogen Electrode Demonstration YouTube Hydrogen Electrode Acid The electrode chosen as the zero voltage (standard electrode) is shown in figure 17.4.1 and is called the standard hydrogen electrode (she). The choice that has been adopted is the standard hydrogen electrode, often abbreviated the s.h.e. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. This page explains the background to standard. Hydrogen Electrode Acid.