What Is A Low Boiling Point . The boiling point is the temperature for a particular liquid to boil at. The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. For example, at a high pressure a. A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric pressure, and vapor pressure. The boiling point of a substance is highly dependent on the environment around the substance. Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The change from a liquid phase to a gaseous phase. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The distance between molecules in a.
from www.slideserve.com
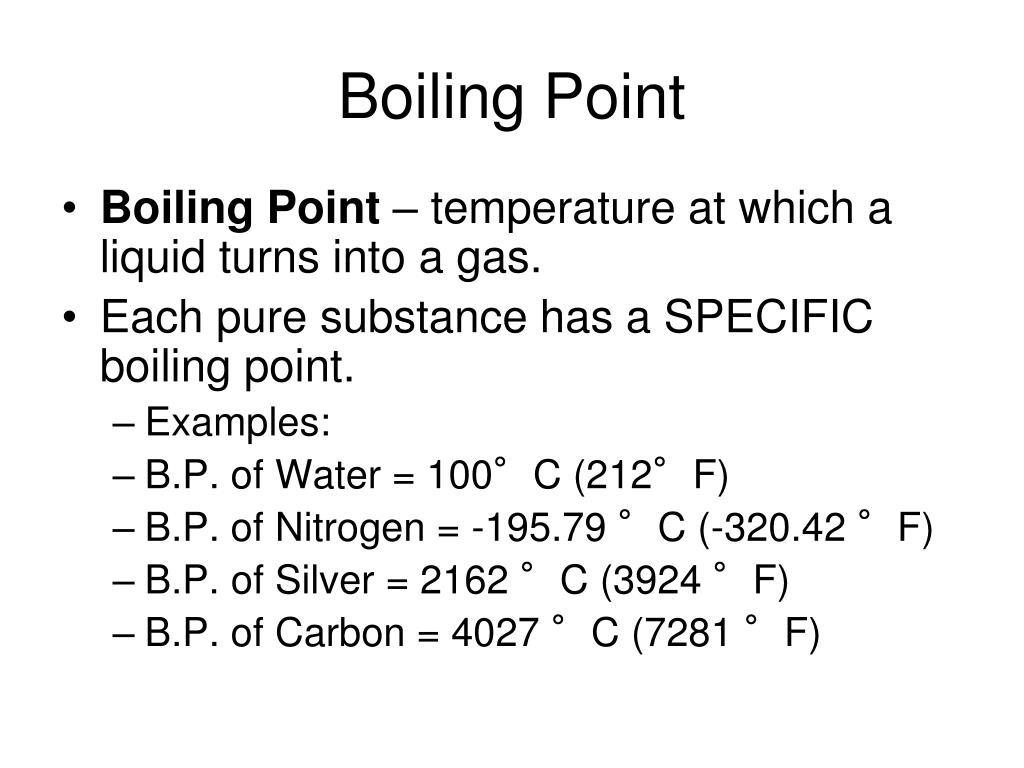
The boiling point is the temperature for a particular liquid to boil at. For example, at a high pressure a. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. The distance between molecules in a. A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric pressure, and vapor pressure. The change from a liquid phase to a gaseous phase. The boiling point of a substance is highly dependent on the environment around the substance. Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur.
PPT Boiling Point Notes PowerPoint Presentation, free download ID
What Is A Low Boiling Point The boiling point of a substance is highly dependent on the environment around the substance. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. For example, at a high pressure a. The boiling point of a substance is highly dependent on the environment around the substance. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The distance between molecules in a. A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric pressure, and vapor pressure. The boiling point is the temperature for a particular liquid to boil at. The change from a liquid phase to a gaseous phase. The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is A Low Boiling Point The boiling point is the temperature for a particular liquid to boil at. The boiling point of a substance is highly dependent on the environment around the substance. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. Boiling is the process by which a liquid turns into a vapor when it is. What Is A Low Boiling Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is A Low Boiling Point Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. The distance between molecules in a. The change from a liquid phase to a gaseous phase. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The boiling point is the temperature for a. What Is A Low Boiling Point.
From www.worksheetsplanet.com
What is Boiling Point What Is A Low Boiling Point Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric pressure, and vapor pressure. The boiling point is the temperature. What Is A Low Boiling Point.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is A Low Boiling Point Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. The change from a liquid phase to a gaseous phase. The boiling point is the temperature for a particular liquid to boil at. The. What Is A Low Boiling Point.
From jsmithmoore.com
Boiling point of ethanol celsius What Is A Low Boiling Point For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. The distance between molecules in a. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the. What Is A Low Boiling Point.
From www.researchgate.net
Ts diagram of a lowboiling working fluid. Download Scientific Diagram What Is A Low Boiling Point Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The change from a liquid phase to a gaseous phase. The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. The distance between molecules in a. A liquid’s boiling point depends upon the. What Is A Low Boiling Point.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Is A Low Boiling Point The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. The change from a liquid phase to a gaseous phase. The boiling point is the temperature for a particular liquid to boil at. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. For example, at a. What Is A Low Boiling Point.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is A Low Boiling Point The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point of a substance is highly dependent on the environment around the substance. The change from a liquid phase to a gaseous. What Is A Low Boiling Point.
From byjus.com
Electrovalent compounds have low melting point low boiling point What Is A Low Boiling Point A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric pressure, and vapor pressure. The change from a liquid phase to a gaseous phase. The boiling point of a substance is highly dependent on the environment around the substance. The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. Note that if. What Is A Low Boiling Point.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy What Is A Low Boiling Point Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. The boiling point is the temperature for a particular liquid to boil at. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric. What Is A Low Boiling Point.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is A Low Boiling Point For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. Boiling is the process by which a. What Is A Low Boiling Point.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube What Is A Low Boiling Point The change from a liquid phase to a gaseous phase. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. For example, at a high pressure a. The distance between molecules in a. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. For. What Is A Low Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point Practice Problems Chemistry Steps What Is A Low Boiling Point The change from a liquid phase to a gaseous phase. The distance between molecules in a. The boiling point of a substance is highly dependent on the environment around the substance. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point, temperature at which the pressure exerted by. What Is A Low Boiling Point.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Is A Low Boiling Point The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. The boiling point of a substance is highly dependent on the environment around the substance. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point is the temperature for a particular. What Is A Low Boiling Point.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID What Is A Low Boiling Point A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric pressure, and vapor pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. The boiling point of a substance is highly. What Is A Low Boiling Point.
From www.chemicals.co.uk
A Level Chemistry Revision Organic Chemistry Alcohols What Is A Low Boiling Point The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. The change from a liquid phase to a gaseous phase. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The lower vapor pressure corresponds to a lower boiling point, and therefore the water. What Is A Low Boiling Point.
From www.youtube.com
Revision chemistry why ammonia has a low boiling point YouTube What Is A Low Boiling Point The boiling point of a substance is highly dependent on the environment around the substance. Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric pressure, and vapor pressure. Boiling point, temperature at which the pressure exerted by the surroundings. What Is A Low Boiling Point.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID1624745 What Is A Low Boiling Point Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The boiling point is the temperature for a particular liquid to boil at. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The lower vapor pressure corresponds to a lower. What Is A Low Boiling Point.
From www.slideserve.com
PPT Chapter 13 continued… PowerPoint Presentation, free download ID What Is A Low Boiling Point Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The boiling point is the temperature for a particular liquid to boil at. The change from a liquid phase to a gaseous phase. The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. For. What Is A Low Boiling Point.
From www.slideserve.com
PPT Properties and Changes in Matter PowerPoint Presentation, free What Is A Low Boiling Point Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. A liquid’s boiling point depends upon. What Is A Low Boiling Point.
From www.youtube.com
What is Boiling Point Concept of Boiling Point Boiling Point What Is A Low Boiling Point The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. The distance between molecules in a. A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric pressure, and vapor pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The lower vapor. What Is A Low Boiling Point.
From www.slideserve.com
PPT Properties of Ionic, Covalent, and Metallic Compounds PowerPoint What Is A Low Boiling Point The distance between molecules in a. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The boiling point of a substance is highly dependent on the environment around the substance. For example, at a. What Is A Low Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is A Low Boiling Point Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The change from a liquid phase to a gaseous phase. Note that if cooling had been applied to the liquid on the bottom,. What Is A Low Boiling Point.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff What Is A Low Boiling Point For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. For example, at a high pressure a. The boiling point of a substance is highly dependent on the environment around the substance. Note that if cooling. What Is A Low Boiling Point.
From www.slideshare.net
Chem matters ch7_covalent_bonding What Is A Low Boiling Point The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. The distance between molecules in a. A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric pressure, and vapor pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The lower vapor. What Is A Low Boiling Point.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Is A Low Boiling Point The melting points of crystalline solids cannot be categorized in as simple a fashion as boiling points. A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric pressure, and vapor pressure. The boiling point is the temperature for a particular liquid to boil at. Boiling is the process by which a liquid turns into a vapor when it is. What Is A Low Boiling Point.
From mavink.com
Melting And Boiling Point Chart What Is A Low Boiling Point For example, at a high pressure a. A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric pressure, and vapor pressure. The distance between molecules in a. Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. For example, the boiling point for water, at a pressure of 1 atm,. What Is A Low Boiling Point.
From www.differencebetween.com
Difference Between Boiling Point and Melting Point Compare the What Is A Low Boiling Point For example, at a high pressure a. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The boiling point of a substance is highly dependent on the environment around the substance. Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. Boiling point,. What Is A Low Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Is A Low Boiling Point A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric pressure, and vapor pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The melting points of crystalline solids. What Is A Low Boiling Point.
From refrigeratorsreviewed.com
Why Boiling Point Of Refrigerant Should Be Low Explained What Is A Low Boiling Point The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. The change from a liquid phase to a gaseous phase. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. Note that if cooling had been applied to the liquid on the bottom, these subsequent. What Is A Low Boiling Point.
From facts.net
20 Fascinating Facts About Boiling Point Elevation What Is A Low Boiling Point The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. The change from a liquid phase to a gaseous phase. The boiling point of a substance is highly dependent on the environment around the substance. For example, at a high pressure a. Note that if cooling had been applied to the liquid on the. What Is A Low Boiling Point.
From infogram.com
Top 10 Elements with the lowest boiling point Infogram What Is A Low Boiling Point Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The boiling point of a substance is highly dependent on the environment around the substance. For example, at a high pressure a. The. What Is A Low Boiling Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is A Low Boiling Point The change from a liquid phase to a gaseous phase. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. A liquid’s boiling point depends upon the liquid ‘s temperature, atmospheric pressure, and vapor. What Is A Low Boiling Point.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Is A Low Boiling Point The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. The change from a liquid. What Is A Low Boiling Point.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is A Low Boiling Point For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The change from a liquid phase to a gaseous phase. The distance between molecules in a. For example, at a high pressure a. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point.. What Is A Low Boiling Point.