Q'' Heat Transfer . Where m is the mass of the substance and δ t is the change in its temperature, in units of celsius or. The equation for heat transfer q is. The quantitative relationship between heat transfer and temperature change contains all three factors: Q = m c δ t, 11.7. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and. Q = heat transfer (w (j/s), btu/h) u = overall heat transfer coefficient (w/(m 2 k), btu/(ft 2 h o f)). Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. A = wall area (m. Q = u a dt (1) where. The energy transfer is always from higher temperature to lower. Heat transfer through a surface like a wall can be calculated as. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The heat flux can be ˙q determined by dividing the heat. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q.
from www.youtube.com
The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The quantitative relationship between heat transfer and temperature change contains all three factors: Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. Heat transfer through a surface like a wall can be calculated as. Q = heat transfer (w (j/s), btu/h) u = overall heat transfer coefficient (w/(m 2 k), btu/(ft 2 h o f)). The equation for heat transfer q is. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and. The energy transfer is always from higher temperature to lower. Q = m c δ t, 11.7.
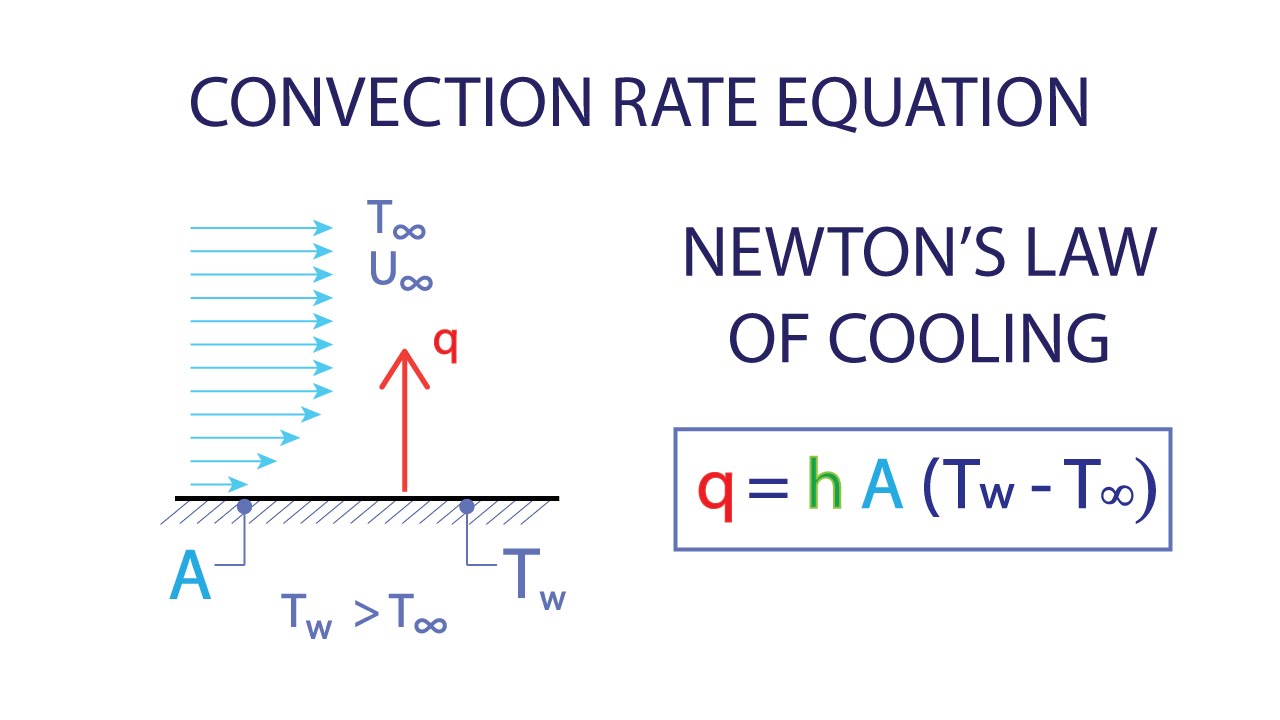
Heat Transfer L2 p2 Convection Rate Equation Newton's Law of
Q'' Heat Transfer The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. A = wall area (m. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and. The equation for heat transfer q is. Q = heat transfer (w (j/s), btu/h) u = overall heat transfer coefficient (w/(m 2 k), btu/(ft 2 h o f)). Q = u a dt (1) where. The quantitative relationship between heat transfer and temperature change contains all three factors: Where m is the mass of the substance and δ t is the change in its temperature, in units of celsius or. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The heat flux can be ˙q determined by dividing the heat. The energy transfer is always from higher temperature to lower. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. Heat transfer through a surface like a wall can be calculated as. Q = m c δ t, 11.7.
From www.youtube.com
Heat Transfer YouTube Q'' Heat Transfer Q = u a dt (1) where. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and. A = wall area (m. The equation for heat transfer q is. Q. Q'' Heat Transfer.
From mungfali.com
Heat Transfer Coefficient Equation Q'' Heat Transfer The energy transfer is always from higher temperature to lower. Q = m c δ t, 11.7. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase.. Q'' Heat Transfer.
From engineerexcel.com
Heat Loss from Pipes A Complete Guide EngineerExcel Q'' Heat Transfer Q = m c δ t, 11.7. Heat transfer through a surface like a wall can be calculated as. Q = u a dt (1) where. The equation for heat transfer q is. The energy transfer is always from higher temperature to lower. Where m is the mass of the substance and δ t is the change in its temperature,. Q'' Heat Transfer.
From www.slideshare.net
11 Heat Transfer Q'' Heat Transfer The energy transfer is always from higher temperature to lower. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. A. Q'' Heat Transfer.
From www.simscale.com
heat transfer by pchand_nag SimScale Q'' Heat Transfer Q = heat transfer (w (j/s), btu/h) u = overall heat transfer coefficient (w/(m 2 k), btu/(ft 2 h o f)). Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and. Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. Q =. Q'' Heat Transfer.
From www.youtube.com
Heat Transfer L2 p2 Convection Rate Equation Newton's Law of Q'' Heat Transfer The heat flux can be ˙q determined by dividing the heat. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. The quantitative relationship between heat transfer and temperature change contains all three factors: Where m is the mass of the substance and δ t is the change in. Q'' Heat Transfer.
From www.studyiq.com
Heat Transfer Types, Definition, Convection, Radiation, Conduction Q'' Heat Transfer Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. The energy transfer is always from higher temperature to lower. A = wall area (m. The heat flux can be ˙q determined by dividing the heat. Q = m c δ t, 11.7. The symbol c stands for the. Q'' Heat Transfer.
From mavink.com
Heat Transfer Coefficient Units Q'' Heat Transfer The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The equation for heat transfer q is. The heat flux can be ˙q determined by dividing the heat. Where m is the mass of the substance and δ t is the change in its temperature, in units of. Q'' Heat Transfer.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Q'' Heat Transfer The equation for heat transfer q is. Where m is the mass of the substance and δ t is the change in its temperature, in units of celsius or. The heat flux can be ˙q determined by dividing the heat. Q = u a dt (1) where. Heat transfer is the movement of heat due to a temperature difference between. Q'' Heat Transfer.
From heattransferkarikuse.blogspot.com
Heat Transfer Heat Transfer Q Equation Q'' Heat Transfer A = wall area (m. Q = heat transfer (w (j/s), btu/h) u = overall heat transfer coefficient (w/(m 2 k), btu/(ft 2 h o f)). Where m is the mass of the substance and δ t is the change in its temperature, in units of celsius or. The symbol c stands for the specific heat (also called “ specific. Q'' Heat Transfer.
From steticlounge.com.br
Ways Of Heat Transfer Q'' Heat Transfer Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. The energy transfer is always from higher temperature to lower. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. Heat transfer through a surface like a. Q'' Heat Transfer.
From www.slideshare.net
11 Heat Transfer Q'' Heat Transfer Q = u a dt (1) where. The energy transfer is always from higher temperature to lower. The equation for heat transfer q is. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Q = heat transfer (w (j/s), btu/h) u. Q'' Heat Transfer.
From pharmacalc.blogspot.com
Overall Heat Transfer CoEfficient Calculation Pharma Engineering Q'' Heat Transfer Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of. Q'' Heat Transfer.
From ar.inspiredpencil.com
Heat Transfer Equation Q'' Heat Transfer Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Q = heat transfer (w (j/s), btu/h) u = overall heat transfer coefficient (w/(m 2 k), btu/(ft 2 h o f)). The equation for heat transfer q is. Heat transfer is the movement of heat due to a temperature. Q'' Heat Transfer.
From www.engineeringtoolbox.com
Convective Heat Transfer Q'' Heat Transfer Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Q = m c δ t, 11.7. The equation for heat transfer q is. The energy transfer is always from higher temperature to lower. The quantitative relationship between heat transfer and temperature change contains all three factors: Heat transfer. Q'' Heat Transfer.
From www.wikihow.com
17 Ways to Solve a Basic Heat Transfer Problem in Thermodynamics Q'' Heat Transfer Heat transfer through a surface like a wall can be calculated as. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Where m is the mass of the substance and δ t is the change in its temperature, in units of celsius or. The heat flux can be. Q'' Heat Transfer.
From www.simscale.com
Heat Transfer SHS by fluxstructural SimScale Q'' Heat Transfer Q = u a dt (1) where. Where m is the mass of the substance and δ t is the change in its temperature, in units of celsius or. The energy transfer is always from higher temperature to lower. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and. The quantitative. Q'' Heat Transfer.
From heattransferkarikuse.blogspot.com
Heat Transfer Heat Transfer Q Equation Q'' Heat Transfer Q = heat transfer (w (j/s), btu/h) u = overall heat transfer coefficient (w/(m 2 k), btu/(ft 2 h o f)). The heat flux can be ˙q determined by dividing the heat. Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. Q = mcδt, where q is the symbol for heat. Q'' Heat Transfer.
From www.simscale.com
Test Heat Transfer by gdutli SimScale Q'' Heat Transfer The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. Heat transfer through a surface like a wall can be calculated as. The equation for heat transfer q is. Q = m c δ t, 11.7. The energy transfer is always from higher temperature to lower. A =. Q'' Heat Transfer.
From www.simscale.com
Heat Transfer by honourister SimScale Q'' Heat Transfer The quantitative relationship between heat transfer and temperature change contains all three factors: The equation for heat transfer q is. Where m is the mass of the substance and δ t is the change in its temperature, in units of celsius or. A = wall area (m. The heat flux can be ˙q determined by dividing the heat. Q =. Q'' Heat Transfer.
From studylib.net
What is Heat Transfer? Q'' Heat Transfer Q = u a dt (1) where. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. The heat flux can be ˙q determined by dividing the heat. The symbol c. Q'' Heat Transfer.
From www.simscale.com
Heat Transfer assignment by hsaner SimScale Q'' Heat Transfer The equation for heat transfer q is. Where m is the mass of the substance and δ t is the change in its temperature, in units of celsius or. A = wall area (m. Q = m c δ t, 11.7. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the. Q'' Heat Transfer.
From www.slideshare.net
Basic Heat Transfer Concepts Q'' Heat Transfer Where m is the mass of the substance and δ t is the change in its temperature, in units of celsius or. Q = u a dt (1) where. Heat transfer through a surface like a wall can be calculated as. A = wall area (m. Q = m c δ t, 11.7. The energy transfer is always from higher. Q'' Heat Transfer.
From www.slideserve.com
PPT SECTION 1 HEAT TRANSFER ANALYSIS PowerPoint Presentation, free Q'' Heat Transfer The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. Where m is the mass of the substance and δ t is the change in its temperature, in units of celsius or. A = wall area (m. The energy transfer is always from higher temperature to lower. Heat. Q'' Heat Transfer.
From www.numerade.com
SOLVED P5.16 Convection heat transfer data are often reported as a Q'' Heat Transfer Q = u a dt (1) where. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. The energy transfer is always from higher temperature to lower. Q = m. Q'' Heat Transfer.
From www.alamy.com
Methods of heat transfer Stock Vector Image & Art Alamy Q'' Heat Transfer Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance, and. Heat transfer through a surface like a wall can be calculated as. The heat flux can be ˙q determined by dividing the heat. Q = m c δ t, 11.7. The equation for heat transfer q is. A = wall area. Q'' Heat Transfer.
From www.madebyteachers.com
Heat Transfer Calorimetry q = mCT First Law of Thermodynamics Worksheet Q'' Heat Transfer The energy transfer is always from higher temperature to lower. Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Where m is the. Q'' Heat Transfer.
From learningmole.com
Heat Transfer LearningMole Q'' Heat Transfer Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. A = wall area (m. The quantitative relationship between heat transfer and temperature change contains all three factors: Where m is the mass of the substance and δ t is the change. Q'' Heat Transfer.
From www.climate-debate.com
Greenhouse Gases Do NOT Violate The StefanBoltzmann Law (page 8) Q'' Heat Transfer Q = heat transfer (w (j/s), btu/h) u = overall heat transfer coefficient (w/(m 2 k), btu/(ft 2 h o f)). Q = u a dt (1) where. The quantitative relationship between heat transfer and temperature change contains all three factors: Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the. Q'' Heat Transfer.
From mungfali.com
Heat Transfer Coefficient Equation Q'' Heat Transfer Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. A = wall area (m. Q = heat transfer (w (j/s), btu/h) u =. Q'' Heat Transfer.
From www.slidemake.com
Einstein Theory Of Specific Heat Presentation Q'' Heat Transfer The equation for heat transfer q is. The quantitative relationship between heat transfer and temperature change contains all three factors: Q = m c δ t, 11.7. Where m is the mass of the substance and δ t is the change in its temperature, in units of celsius or. Heat transfer is the movement of heat due to a temperature. Q'' Heat Transfer.
From www.simscale.com
heat transfer by ravensky SimScale Q'' Heat Transfer Heat transfer through a surface like a wall can be calculated as. A = wall area (m. Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. The energy transfer is always from higher temperature to lower. Where m is the mass of the substance and δ t is the change in. Q'' Heat Transfer.
From www.slideshare.net
11 Heat Transfer Q'' Heat Transfer Heat transfer through a surface like a wall can be calculated as. A = wall area (m. The energy transfer is always from higher temperature to lower. Sometimes it is important to determine the heat transfer rate per unit area, or heat flux, which has the symbol q. Q = heat transfer (w (j/s), btu/h) u = overall heat transfer. Q'' Heat Transfer.
From www.youtube.com
Example of Heat Transfer calculation part 1 YouTube Q'' Heat Transfer The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The energy transfer is always from higher temperature to lower. Q = u a dt (1) where. Q = heat transfer (w (j/s), btu/h) u = overall heat transfer coefficient (w/(m 2 k), btu/(ft 2 h o f)).. Q'' Heat Transfer.
From answermediabrandt.z19.web.core.windows.net
Heat Transfer Specific Heat Problems Worksheet Q'' Heat Transfer Heat transfer through a surface like a wall can be calculated as. The equation for heat transfer q is. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. A = wall area (m. Q = heat transfer (w (j/s), btu/h) u. Q'' Heat Transfer.