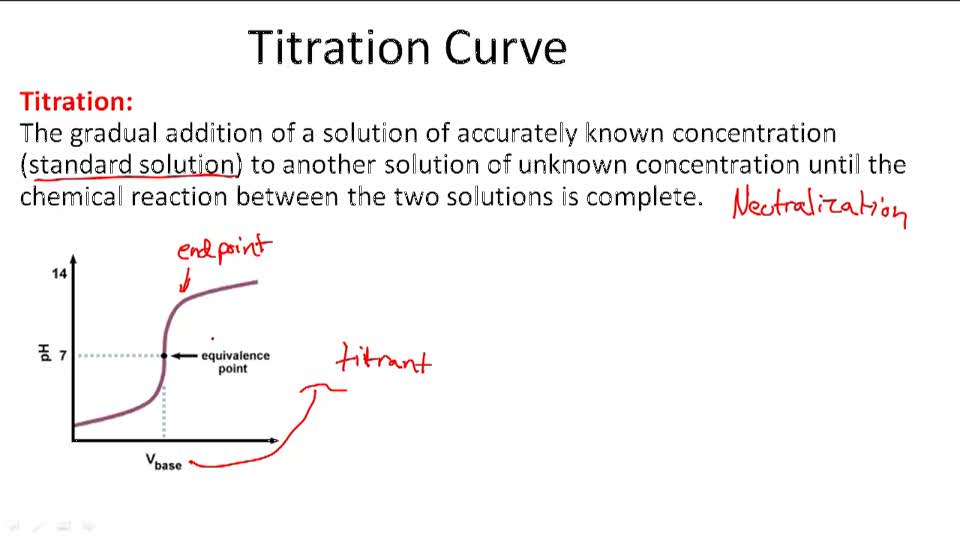
How To Write A Titration Equation . By the end of this section, you will be able to: The volumes of acids and alkali solutions that react with each other can be. You will also learn about. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the.
from www.ck12.org
The volumes of acids and alkali solutions that react with each other can be. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. By the end of this section, you will be able to: You will also learn about. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one.
Titration (Calculations) Overview ( Video ) Chemistry CK12
How To Write A Titration Equation You will also learn about. The volumes of acids and alkali solutions that react with each other can be. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. By the end of this section, you will be able to: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. You will also learn about. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one.
From www.chegg.com
Solved a. Write the chemical equation for the titration How To Write A Titration Equation — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The volumes of acids and alkali solutions that react with each other can be. You will also learn about. At the equivalence point in a neutralization, the moles of acid are equal to. How To Write A Titration Equation.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube How To Write A Titration Equation calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. At the equivalence. How To Write A Titration Equation.
From www.slideserve.com
PPT Neutralization & Titration PowerPoint Presentation, free download How To Write A Titration Equation — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The volumes of acids and alkali solutions that react with each other can be. You will also learn about. By the end of this section, you will be able to: In this tutorial,. How To Write A Titration Equation.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube How To Write A Titration Equation The volumes of acids and alkali solutions that react with each other can be. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. At the equivalence point in a neutralization, the moles of acid are equal to the moles. How To Write A Titration Equation.
From acid-base-titration.blogspot.com
Acid Base Titration Titrating Weak Acid With Strong Base How To Write A Titration Equation By the end of this section, you will be able to: — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The volumes of acids and alkali solutions that react with each other can be. calculate the ph for the strong acid/strong. How To Write A Titration Equation.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube How To Write A Titration Equation calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. By the end of this section, you will be able to: You. How To Write A Titration Equation.
From missmeyerscience.blogspot.com
Miss Meyer's Science Site Titration Calculations How To Write A Titration Equation calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. In this tutorial,. How To Write A Titration Equation.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube How To Write A Titration Equation By the end of this section, you will be able to: The volumes of acids and alkali solutions that react with each other can be. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — a titration is a volumetric technique in which a solution of one reactant (the titrant). How To Write A Titration Equation.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 How To Write A Titration Equation calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. The volumes of acids and alkali solutions that react with each other can be. In this tutorial, you will learn about titration curves, titration analysis and the steps required to. How To Write A Titration Equation.
From theedge.com.hk
Chemistry How To Titration The Edge How To Write A Titration Equation calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You will also learn about. At the equivalence point in a neutralization,. How To Write A Titration Equation.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 How To Write A Titration Equation — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The volumes of acids and alkali solutions that react with each other can be. By the end of this section, you will be able to: At the equivalence point in a neutralization, the. How To Write A Titration Equation.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube How To Write A Titration Equation — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. By the end of this section, you will be able to: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. In this tutorial, you. How To Write A Titration Equation.
From ar.inspiredpencil.com
Titration Equation How To Write A Titration Equation In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a. How To Write A Titration Equation.
From www.youtube.com
Solving AcidBase Titration Problems YouTube How To Write A Titration Equation At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. You will also learn about. By the end of this section, you will be able. How To Write A Titration Equation.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube How To Write A Titration Equation By the end of this section, you will be able to: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. You will also learn about. — a titration is a volumetric technique in which a solution of one. How To Write A Titration Equation.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe How To Write A Titration Equation You will also learn about. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. By the end of this section, you will be able to: In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform. How To Write A Titration Equation.
From learningschoolmysticis9.z22.web.core.windows.net
How To Do Titrations In Chemistry How To Write A Titration Equation At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. — a titration is a volumetric technique in which a solution. How To Write A Titration Equation.
From www.youtube.com
CHEM 101 Titration of Unknown Triprotic Acid YouTube How To Write A Titration Equation In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a. How To Write A Titration Equation.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions How To Write A Titration Equation By the end of this section, you will be able to: In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. The volumes of acids and alkali solutions that react with each other can be. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno. How To Write A Titration Equation.
From mavink.com
Acid Base Titration Equation How To Write A Titration Equation The volumes of acids and alkali solutions that react with each other can be. In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes. How To Write A Titration Equation.
From www.youtube.com
Titration Calculations YouTube How To Write A Titration Equation In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You will also learn about. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq). How To Write A Titration Equation.
From www.numerade.com
SOLVED Write the equation for the titration of Ca2+ with EDTA. How To Write A Titration Equation In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. You will also learn about. calculate the ph for the strong acid/strong base titration. How To Write A Titration Equation.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube How To Write A Titration Equation By the end of this section, you will be able to: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. You will also learn about. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. How To Write A Titration Equation.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube How To Write A Titration Equation By the end of this section, you will be able to: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. In. How To Write A Titration Equation.
From www.youtube.com
Titration calculations Part 1 YouTube How To Write A Titration Equation At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The volumes of acids and alkali solutions that react with each other can be. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes. How To Write A Titration Equation.
From www.youtube.com
Modeling a Titration Example with an Equation (Including a Review of How To Write A Titration Equation By the end of this section, you will be able to: — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. You will also learn. How To Write A Titration Equation.
From slideplayer.com
Titration. ppt download How To Write A Titration Equation You will also learn about. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. calculate the ph for the strong acid/strong base titration. How To Write A Titration Equation.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube How To Write A Titration Equation calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. By the end of this section, you will be able to: — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added. How To Write A Titration Equation.
From www.numerade.com
SOLVED The balanced equation for the titration of oxalic acid with How To Write A Titration Equation You will also learn about. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. The volumes of acids and alkali solutions that react with each other can be. By the end of this section, you will be able to:. How To Write A Titration Equation.
From www.youtube.com
Basic Titrations Chemistry Tutorial YouTube How To Write A Titration Equation At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. You will also learn about. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The volumes of acids and alkali solutions that react with. How To Write A Titration Equation.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 How To Write A Titration Equation — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. You will also learn about. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes. How To Write A Titration Equation.
From www.youtube.com
G11 Characteristics of Redox Titration Equation YouTube How To Write A Titration Equation The volumes of acids and alkali solutions that react with each other can be. You will also learn about. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh. How To Write A Titration Equation.
From exosqdnkw.blob.core.windows.net
Titration Calculations A Level Aqa at John Walling blog How To Write A Titration Equation At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. — a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. You will also learn about. The volumes of acids and alkali solutions that react with. How To Write A Titration Equation.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 How To Write A Titration Equation In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You will also learn about. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. By the end of this section, you will be able to: The volumes of acids and alkali solutions that. How To Write A Titration Equation.
From www.youtube.com
Calculations for Titration of Weak Base with Strong Acid, initial pH How To Write A Titration Equation By the end of this section, you will be able to: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You. How To Write A Titration Equation.