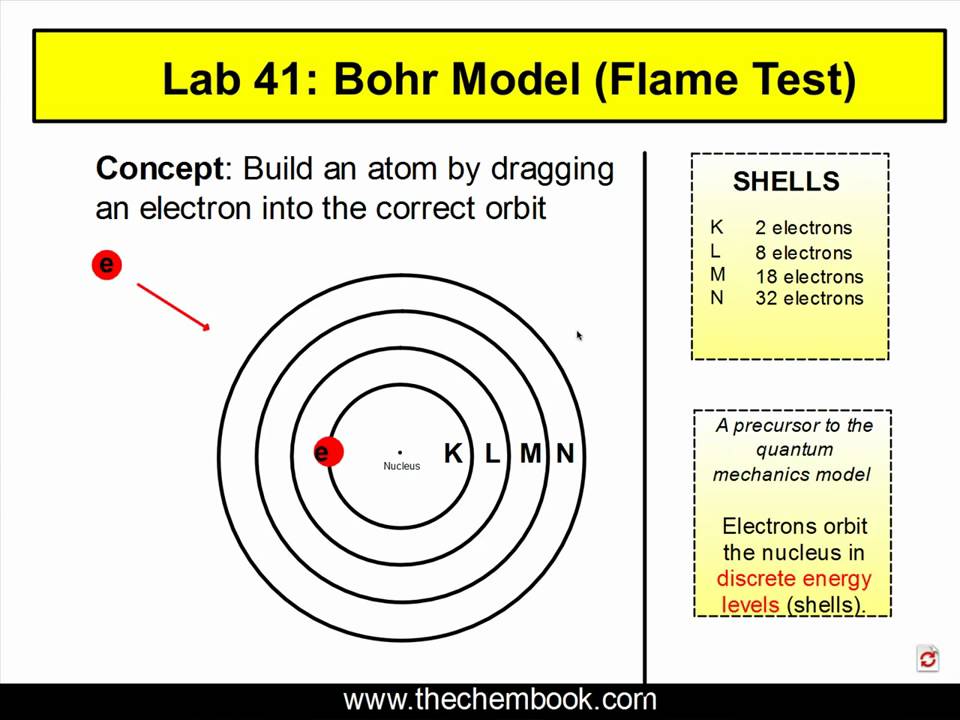
In The Flame Test Lab How Are Electrons Excited . When placed in the flame, the metals. in this part of the experiment, electrons are excited by absorbing the heat from the bunsen burner flame, which allowed them. when the electron has been promoted to a higher energy level, the atom is said to be in an excited state. — so, how does electromagnetic radiation relate to flame tests? When the electrons are excited, they move to the next orbital, or energy level. Well, when an atom (or ion) absorbs energy, its electrons can make. Heating the atoms in the flame excites the electrons. in this experiment, different unknown metals are burned and students will identify the metal based on the color of the flame. — in this experiment, the metal cations in the solutions were initially in the (ground, excited) state. — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts of excited states of the. — as we know that when an atom or ion is excited by heating to high temperatures, the electrons are promoted.
from www.youtube.com
Heating the atoms in the flame excites the electrons. in this part of the experiment, electrons are excited by absorbing the heat from the bunsen burner flame, which allowed them. — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts of excited states of the. When the electrons are excited, they move to the next orbital, or energy level. Well, when an atom (or ion) absorbs energy, its electrons can make. in this experiment, different unknown metals are burned and students will identify the metal based on the color of the flame. When placed in the flame, the metals. when the electron has been promoted to a higher energy level, the atom is said to be in an excited state. — as we know that when an atom or ion is excited by heating to high temperatures, the electrons are promoted. — in this experiment, the metal cations in the solutions were initially in the (ground, excited) state.
Bohr Model Flame Test Explanation.mp4 YouTube
In The Flame Test Lab How Are Electrons Excited — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts of excited states of the. in this part of the experiment, electrons are excited by absorbing the heat from the bunsen burner flame, which allowed them. Heating the atoms in the flame excites the electrons. When the electrons are excited, they move to the next orbital, or energy level. Well, when an atom (or ion) absorbs energy, its electrons can make. When placed in the flame, the metals. — in this experiment, the metal cations in the solutions were initially in the (ground, excited) state. in this experiment, different unknown metals are burned and students will identify the metal based on the color of the flame. — as we know that when an atom or ion is excited by heating to high temperatures, the electrons are promoted. — so, how does electromagnetic radiation relate to flame tests? — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts of excited states of the. when the electron has been promoted to a higher energy level, the atom is said to be in an excited state.
From www.flinnsci.ca
Flame Tests Atomic Emission and Electron Energy Levels—ChemTopic™ Lab In The Flame Test Lab How Are Electrons Excited When the electrons are excited, they move to the next orbital, or energy level. in this experiment, different unknown metals are burned and students will identify the metal based on the color of the flame. When placed in the flame, the metals. — in this experiment, the metal cations in the solutions were initially in the (ground, excited). In The Flame Test Lab How Are Electrons Excited.
From studylib.net
Flame Tests Lab In The Flame Test Lab How Are Electrons Excited — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts of excited states of the. When placed in the flame, the metals. Well, when an atom (or ion) absorbs energy, its electrons can make. in this experiment, different unknown metals. In The Flame Test Lab How Are Electrons Excited.
From studylib.net
FLAME TEST LAB PROCEDURE In The Flame Test Lab How Are Electrons Excited — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts of excited states of the. When the electrons are excited, they move to the next orbital, or energy level. — in this experiment, the metal cations in the solutions were. In The Flame Test Lab How Are Electrons Excited.
From worksheetscairpe4d.z21.web.core.windows.net
Procedure Of Flame Test In The Flame Test Lab How Are Electrons Excited Heating the atoms in the flame excites the electrons. — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts of excited states of the. — in this experiment, the metal cations in the solutions were initially in the (ground, excited). In The Flame Test Lab How Are Electrons Excited.
From labquiz.netlify.app
Chemistry flame test lab report labquiz In The Flame Test Lab How Are Electrons Excited in this experiment, different unknown metals are burned and students will identify the metal based on the color of the flame. — as we know that when an atom or ion is excited by heating to high temperatures, the electrons are promoted. Well, when an atom (or ion) absorbs energy, its electrons can make. when the electron. In The Flame Test Lab How Are Electrons Excited.
From www.webexhibits.org
Flame tests Causes of Color In The Flame Test Lab How Are Electrons Excited — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts of excited states of the. in this experiment, different unknown metals are burned and students will identify the metal based on the color of the flame. When the electrons are. In The Flame Test Lab How Are Electrons Excited.
From studylib.net
Flame Tests In The Flame Test Lab How Are Electrons Excited in this part of the experiment, electrons are excited by absorbing the heat from the bunsen burner flame, which allowed them. when the electron has been promoted to a higher energy level, the atom is said to be in an excited state. — a sodium atom in an unexcited state has the structure 1s 2 2s 2. In The Flame Test Lab How Are Electrons Excited.
From www.studocu.com
Roberto Granados Excited Electrons Flame test lab Name Studocu In The Flame Test Lab How Are Electrons Excited Well, when an atom (or ion) absorbs energy, its electrons can make. in this experiment, different unknown metals are burned and students will identify the metal based on the color of the flame. when the electron has been promoted to a higher energy level, the atom is said to be in an excited state. — a sodium. In The Flame Test Lab How Are Electrons Excited.
From chemhonors.blogspot.com
Chemistry Honors Ch 4 Notes through Quantum Model of the Atom; Flame In The Flame Test Lab How Are Electrons Excited Heating the atoms in the flame excites the electrons. — in this experiment, the metal cations in the solutions were initially in the (ground, excited) state. Well, when an atom (or ion) absorbs energy, its electrons can make. When the electrons are excited, they move to the next orbital, or energy level. in this experiment, different unknown metals. In The Flame Test Lab How Are Electrons Excited.
From athensmutualaid.net
Flame Test Lab Chemistry › Athens Mutual Student Corner In The Flame Test Lab How Are Electrons Excited When placed in the flame, the metals. in this part of the experiment, electrons are excited by absorbing the heat from the bunsen burner flame, which allowed them. When the electrons are excited, they move to the next orbital, or energy level. — so, how does electromagnetic radiation relate to flame tests? — a sodium atom in. In The Flame Test Lab How Are Electrons Excited.
From www.animalia-life.club
Flame Test Lab Results In The Flame Test Lab How Are Electrons Excited when the electron has been promoted to a higher energy level, the atom is said to be in an excited state. Well, when an atom (or ion) absorbs energy, its electrons can make. — in this experiment, the metal cations in the solutions were initially in the (ground, excited) state. in this part of the experiment, electrons. In The Flame Test Lab How Are Electrons Excited.
From www.youtube.com
The Flame Test Experiment The Chemistry of Colorful Flames YouTube In The Flame Test Lab How Are Electrons Excited — in this experiment, the metal cations in the solutions were initially in the (ground, excited) state. — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts of excited states of the. in this experiment, different unknown metals are. In The Flame Test Lab How Are Electrons Excited.
From studylib.net
Flame Test Exploration In The Flame Test Lab How Are Electrons Excited Well, when an atom (or ion) absorbs energy, its electrons can make. — so, how does electromagnetic radiation relate to flame tests? — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts of excited states of the. — in. In The Flame Test Lab How Are Electrons Excited.
From www.numerade.com
Prelab Assignment Flame Tests of Metal Cations In this lab, You will In The Flame Test Lab How Are Electrons Excited — so, how does electromagnetic radiation relate to flame tests? — in this experiment, the metal cations in the solutions were initially in the (ground, excited) state. Well, when an atom (or ion) absorbs energy, its electrons can make. — as we know that when an atom or ion is excited by heating to high temperatures, the. In The Flame Test Lab How Are Electrons Excited.
From slideplayer.com
Flame Test Lab. ppt download In The Flame Test Lab How Are Electrons Excited Well, when an atom (or ion) absorbs energy, its electrons can make. Heating the atoms in the flame excites the electrons. in this experiment, different unknown metals are burned and students will identify the metal based on the color of the flame. — in this experiment, the metal cations in the solutions were initially in the (ground, excited). In The Flame Test Lab How Are Electrons Excited.
From www.chegg.com
Solved Flame Test & Spectroscopy Background Electrons are In The Flame Test Lab How Are Electrons Excited — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts of excited states of the. in this part of the experiment, electrons are excited by absorbing the heat from the bunsen burner flame, which allowed them. — so, how. In The Flame Test Lab How Are Electrons Excited.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) In The Flame Test Lab How Are Electrons Excited Well, when an atom (or ion) absorbs energy, its electrons can make. in this experiment, different unknown metals are burned and students will identify the metal based on the color of the flame. Heating the atoms in the flame excites the electrons. in this part of the experiment, electrons are excited by absorbing the heat from the bunsen. In The Flame Test Lab How Are Electrons Excited.
From www.youtube.com
Bohr Model Flame Test Explanation.mp4 YouTube In The Flame Test Lab How Are Electrons Excited in this experiment, different unknown metals are burned and students will identify the metal based on the color of the flame. Heating the atoms in the flame excites the electrons. Well, when an atom (or ion) absorbs energy, its electrons can make. — in this experiment, the metal cations in the solutions were initially in the (ground, excited). In The Flame Test Lab How Are Electrons Excited.
From slideplayer.com
Flame Test Lab. ppt download In The Flame Test Lab How Are Electrons Excited — so, how does electromagnetic radiation relate to flame tests? in this experiment, different unknown metals are burned and students will identify the metal based on the color of the flame. in this part of the experiment, electrons are excited by absorbing the heat from the bunsen burner flame, which allowed them. Well, when an atom (or. In The Flame Test Lab How Are Electrons Excited.
From slideplayer.com
Light emissions minilab Pt 1 Flame test Wrap up ppt download In The Flame Test Lab How Are Electrons Excited Well, when an atom (or ion) absorbs energy, its electrons can make. when the electron has been promoted to a higher energy level, the atom is said to be in an excited state. When the electrons are excited, they move to the next orbital, or energy level. — a sodium atom in an unexcited state has the structure. In The Flame Test Lab How Are Electrons Excited.
From studylib.net
Flame Test Lab In The Flame Test Lab How Are Electrons Excited in this part of the experiment, electrons are excited by absorbing the heat from the bunsen burner flame, which allowed them. Well, when an atom (or ion) absorbs energy, its electrons can make. Heating the atoms in the flame excites the electrons. When placed in the flame, the metals. When the electrons are excited, they move to the next. In The Flame Test Lab How Are Electrons Excited.
From studylib.net
Flame Test Lab In The Flame Test Lab How Are Electrons Excited When placed in the flame, the metals. — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts of excited states of the. — as we know that when an atom or ion is excited by heating to high temperatures, the. In The Flame Test Lab How Are Electrons Excited.
From studylib.net
Flame Tests Lab In The Flame Test Lab How Are Electrons Excited Well, when an atom (or ion) absorbs energy, its electrons can make. — so, how does electromagnetic radiation relate to flame tests? When placed in the flame, the metals. Heating the atoms in the flame excites the electrons. — as we know that when an atom or ion is excited by heating to high temperatures, the electrons are. In The Flame Test Lab How Are Electrons Excited.
From www.alamy.com
Sodium flame test. Positive result of a flame test for sodium (Na In The Flame Test Lab How Are Electrons Excited When placed in the flame, the metals. in this experiment, different unknown metals are burned and students will identify the metal based on the color of the flame. — in this experiment, the metal cations in the solutions were initially in the (ground, excited) state. — as we know that when an atom or ion is excited. In The Flame Test Lab How Are Electrons Excited.
From learningricardo.z19.web.core.windows.net
Flame Test Lab Explanation In The Flame Test Lab How Are Electrons Excited — so, how does electromagnetic radiation relate to flame tests? When the electrons are excited, they move to the next orbital, or energy level. Heating the atoms in the flame excites the electrons. when the electron has been promoted to a higher energy level, the atom is said to be in an excited state. When placed in the. In The Flame Test Lab How Are Electrons Excited.
From www.carolina.com
Flame Test Kit In The Flame Test Lab How Are Electrons Excited Heating the atoms in the flame excites the electrons. — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts of excited states of the. — as we know that when an atom or ion is excited by heating to high. In The Flame Test Lab How Are Electrons Excited.
From slideplayer.com
Flame Test Lab. ppt download In The Flame Test Lab How Are Electrons Excited — in this experiment, the metal cations in the solutions were initially in the (ground, excited) state. — so, how does electromagnetic radiation relate to flame tests? Well, when an atom (or ion) absorbs energy, its electrons can make. in this experiment, different unknown metals are burned and students will identify the metal based on the color. In The Flame Test Lab How Are Electrons Excited.
From webapi.bu.edu
💌 Flame test lab purpose. What is the purpose of the flame lab test In The Flame Test Lab How Are Electrons Excited — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts of excited states of the. in this part of the experiment, electrons are excited by absorbing the heat from the bunsen burner flame, which allowed them. — so, how. In The Flame Test Lab How Are Electrons Excited.
From www.slideserve.com
PPT Chapter 7; Electronic Structure of Atoms PowerPoint Presentation In The Flame Test Lab How Are Electrons Excited in this experiment, different unknown metals are burned and students will identify the metal based on the color of the flame. when the electron has been promoted to a higher energy level, the atom is said to be in an excited state. Well, when an atom (or ion) absorbs energy, its electrons can make. When the electrons are. In The Flame Test Lab How Are Electrons Excited.
From www.youtube.com
Electrons in the Flame Test Lab YouTube In The Flame Test Lab How Are Electrons Excited Well, when an atom (or ion) absorbs energy, its electrons can make. in this part of the experiment, electrons are excited by absorbing the heat from the bunsen burner flame, which allowed them. when the electron has been promoted to a higher energy level, the atom is said to be in an excited state. — as we. In The Flame Test Lab How Are Electrons Excited.
From studylib.net
The Flame Test In The Flame Test Lab How Are Electrons Excited Well, when an atom (or ion) absorbs energy, its electrons can make. When the electrons are excited, they move to the next orbital, or energy level. When placed in the flame, the metals. — so, how does electromagnetic radiation relate to flame tests? — in this experiment, the metal cations in the solutions were initially in the (ground,. In The Flame Test Lab How Are Electrons Excited.
From sciencestuffbyamy.blogspot.com
Amy Brown Science Flame Tests A Favorite Chemistry Lab In The Flame Test Lab How Are Electrons Excited Heating the atoms in the flame excites the electrons. Well, when an atom (or ion) absorbs energy, its electrons can make. in this part of the experiment, electrons are excited by absorbing the heat from the bunsen burner flame, which allowed them. in this experiment, different unknown metals are burned and students will identify the metal based on. In The Flame Test Lab How Are Electrons Excited.
From studylib.net
Flame Test Lab In The Flame Test Lab How Are Electrons Excited in this part of the experiment, electrons are excited by absorbing the heat from the bunsen burner flame, which allowed them. when the electron has been promoted to a higher energy level, the atom is said to be in an excited state. — so, how does electromagnetic radiation relate to flame tests? in this experiment, different. In The Flame Test Lab How Are Electrons Excited.
From sciencelessonsthatrock.com
Chemistry Flame Test Lab Science Lessons That Rock In The Flame Test Lab How Are Electrons Excited Heating the atoms in the flame excites the electrons. in this part of the experiment, electrons are excited by absorbing the heat from the bunsen burner flame, which allowed them. — a sodium atom in an unexcited state has the structure 1s 2 2s 2 2p 6 3s 1, but within the flame there will be all sorts. In The Flame Test Lab How Are Electrons Excited.
From www.youtube.com
Flame Test Lab YouTube In The Flame Test Lab How Are Electrons Excited in this part of the experiment, electrons are excited by absorbing the heat from the bunsen burner flame, which allowed them. When the electrons are excited, they move to the next orbital, or energy level. — in this experiment, the metal cations in the solutions were initially in the (ground, excited) state. — a sodium atom in. In The Flame Test Lab How Are Electrons Excited.