Naoh Khp Titration Lab Report . The same reasons we choose khp as our. chm 115 lab 3 titration: — the titration reaction of khp with naoh is as follows: Sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. You will precisely measure the concentration. — attach the graph to your lab report. in this laboratory exercise you will carry out such a titration to standardize (determine the exact. Standardize naoh/determine impure khp purpose: The solution is then standardized, that is, its. Determine the equivalence point (in ml of naoh) of this ph titration. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. \[\ce{c8h5ko4 (aq) + naoh (aq) → h2o + c8h4nako4 (aq)}\nonumber\] the. khp works as a great primary standard for determination of naoh concentrations.
from www.numerade.com
— the titration reaction of khp with naoh is as follows: The solution is then standardized, that is, its. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. — attach the graph to your lab report. in this laboratory exercise you will carry out such a titration to standardize (determine the exact. chm 115 lab 3 titration: Standardize naoh/determine impure khp purpose: khp works as a great primary standard for determination of naoh concentrations. Determine the equivalence point (in ml of naoh) of this ph titration. The same reasons we choose khp as our.
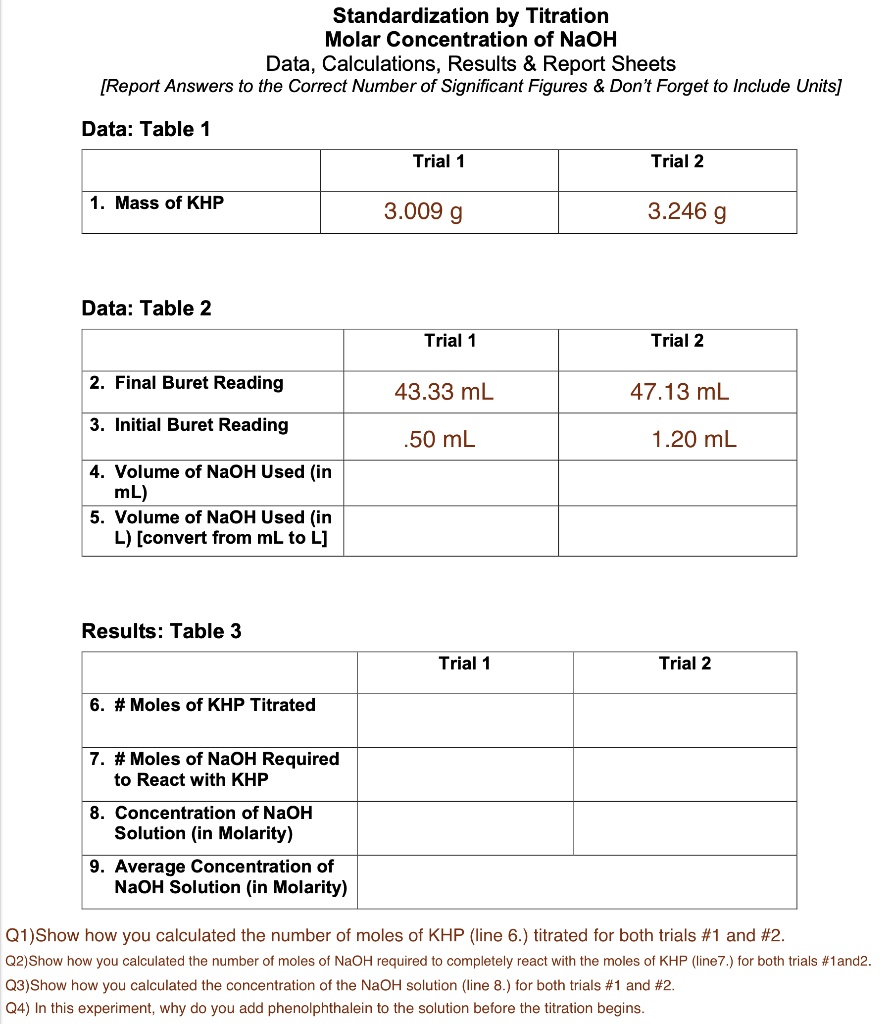
SOLVED Standardization by Titration Molar Concentration of NaOH Data
Naoh Khp Titration Lab Report in this laboratory exercise you will carry out such a titration to standardize (determine the exact. — attach the graph to your lab report. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. — the titration reaction of khp with naoh is as follows: Determine the equivalence point (in ml of naoh) of this ph titration. chm 115 lab 3 titration: Sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. The same reasons we choose khp as our. Standardize naoh/determine impure khp purpose: khp works as a great primary standard for determination of naoh concentrations. \[\ce{c8h5ko4 (aq) + naoh (aq) → h2o + c8h4nako4 (aq)}\nonumber\] the. You will precisely measure the concentration. in this laboratory exercise you will carry out such a titration to standardize (determine the exact. The solution is then standardized, that is, its.
From oneclass.com
OneClass REPORT SHEET EXPERIMENT Titration of Acids and Bases 20 Naoh Khp Titration Lab Report You will precisely measure the concentration. — attach the graph to your lab report. The solution is then standardized, that is, its. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. Determine the equivalence point (in ml of naoh) of this ph titration. khp works as. Naoh Khp Titration Lab Report.
From www.numerade.com
SOLVED Hi, I am writing a titration lab report (KHP and NaOH Naoh Khp Titration Lab Report a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. Determine the equivalence point (in ml of naoh) of this ph titration. The same reasons we choose khp as our. Sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. —. Naoh Khp Titration Lab Report.
From www.studypool.com
SOLUTION Laboratory Report Standardization of NaOH with KHP and Naoh Khp Titration Lab Report chm 115 lab 3 titration: You will precisely measure the concentration. — the titration reaction of khp with naoh is as follows: khp works as a great primary standard for determination of naoh concentrations. Determine the equivalence point (in ml of naoh) of this ph titration. The solution is then standardized, that is, its. a titration. Naoh Khp Titration Lab Report.
From plot.ly
KHP and NaOH Titration Curve line chart made by Kylclk plotly Naoh Khp Titration Lab Report Determine the equivalence point (in ml of naoh) of this ph titration. — attach the graph to your lab report. You will precisely measure the concentration. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. Standardize naoh/determine impure khp purpose: chm 115 lab 3 titration: The. Naoh Khp Titration Lab Report.
From www.scribd.com
Laboratory Plan 1 Standardization of Sodium Hydroxide (Naoh) With Naoh Khp Titration Lab Report khp works as a great primary standard for determination of naoh concentrations. — attach the graph to your lab report. in this laboratory exercise you will carry out such a titration to standardize (determine the exact. Determine the equivalence point (in ml of naoh) of this ph titration. Sodium hydroxide solution of about 0.2 m is prepared. Naoh Khp Titration Lab Report.
From www.youtube.com
CTC 114 Standardization Pre Lab Video Standardization of Sodium Naoh Khp Titration Lab Report The same reasons we choose khp as our. The solution is then standardized, that is, its. — attach the graph to your lab report. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. Standardize naoh/determine impure khp purpose: — the titration reaction of khp with naoh. Naoh Khp Titration Lab Report.
From www.chegg.com
Solved KHP Titration Report Sheet Concentration of NaOH Naoh Khp Titration Lab Report a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. — attach the graph to your lab report. The same reasons we choose khp as our. in this laboratory exercise you will carry out such a titration to standardize (determine the exact. Standardize naoh/determine impure khp purpose:. Naoh Khp Titration Lab Report.
From www.numerade.com
SOLVED Part III Titration Standardization of NaOH solution (finding Naoh Khp Titration Lab Report in this laboratory exercise you will carry out such a titration to standardize (determine the exact. The same reasons we choose khp as our. — attach the graph to your lab report. Sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. Standardize naoh/determine impure khp purpose: a titration experiment. Naoh Khp Titration Lab Report.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Naoh Khp Titration Lab Report — attach the graph to your lab report. The solution is then standardized, that is, its. You will precisely measure the concentration. — the titration reaction of khp with naoh is as follows: \[\ce{c8h5ko4 (aq) + naoh (aq) → h2o + c8h4nako4 (aq)}\nonumber\] the. Standardize naoh/determine impure khp purpose: Sodium hydroxide solution of about 0.2 m is prepared. Naoh Khp Titration Lab Report.
From www.studocu.com
PostLab Questions of Titration of KHP with NaOH solution Post Lab Naoh Khp Titration Lab Report The same reasons we choose khp as our. khp works as a great primary standard for determination of naoh concentrations. chm 115 lab 3 titration: You will precisely measure the concentration. — attach the graph to your lab report. in this laboratory exercise you will carry out such a titration to standardize (determine the exact. . Naoh Khp Titration Lab Report.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Naoh Khp Titration Lab Report Determine the equivalence point (in ml of naoh) of this ph titration. — attach the graph to your lab report. in this laboratory exercise you will carry out such a titration to standardize (determine the exact. You will precisely measure the concentration. a titration experiment is one where one attempts to determine the concentration of a sample. Naoh Khp Titration Lab Report.
From www.numerade.com
SOLVED Text Name Desk Date Laboratory Instructor REPORT SHEET Naoh Khp Titration Lab Report — the titration reaction of khp with naoh is as follows: Sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. khp works as a great primary standard for determination of naoh concentrations. Determine the equivalence point (in ml of naoh) of this ph titration. The solution is then standardized, that. Naoh Khp Titration Lab Report.
From www.studocu.com
Report 1 full Experiment on titration of NaOH and KHP Table of Naoh Khp Titration Lab Report You will precisely measure the concentration. \[\ce{c8h5ko4 (aq) + naoh (aq) → h2o + c8h4nako4 (aq)}\nonumber\] the. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. in this laboratory exercise you will carry out such a titration to standardize (determine the exact. — attach the graph. Naoh Khp Titration Lab Report.
From www.numerade.com
SOLVED Standardization by Titration Molar Concentration of NaOH Data Naoh Khp Titration Lab Report The same reasons we choose khp as our. — attach the graph to your lab report. Standardize naoh/determine impure khp purpose: Determine the equivalence point (in ml of naoh) of this ph titration. in this laboratory exercise you will carry out such a titration to standardize (determine the exact. Sodium hydroxide solution of about 0.2 m is prepared. Naoh Khp Titration Lab Report.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Naoh Khp Titration Lab Report \[\ce{c8h5ko4 (aq) + naoh (aq) → h2o + c8h4nako4 (aq)}\nonumber\] the. chm 115 lab 3 titration: khp works as a great primary standard for determination of naoh concentrations. Sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. The same reasons we choose khp as our. You will precisely measure the. Naoh Khp Titration Lab Report.
From www.chegg.com
Solved TITRATION ⋅ STANDARDIZATION OF SODIUM HYDROXIDE Naoh Khp Titration Lab Report You will precisely measure the concentration. Determine the equivalence point (in ml of naoh) of this ph titration. chm 115 lab 3 titration: khp works as a great primary standard for determination of naoh concentrations. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. The same. Naoh Khp Titration Lab Report.
From www.numerade.com
SOLVED Name Desk Date Laboratory Instructor REPORT SHEET Titration of Naoh Khp Titration Lab Report — attach the graph to your lab report. in this laboratory exercise you will carry out such a titration to standardize (determine the exact. The solution is then standardized, that is, its. khp works as a great primary standard for determination of naoh concentrations. Sodium hydroxide solution of about 0.2 m is prepared in order to be. Naoh Khp Titration Lab Report.
From www.numerade.com
SOLVED KHP Titration Report Sheet Concentration of NaOH solution 0.148 Naoh Khp Titration Lab Report — attach the graph to your lab report. khp works as a great primary standard for determination of naoh concentrations. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. Determine the equivalence point (in ml of naoh) of this ph titration. chm 115 lab 3. Naoh Khp Titration Lab Report.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Naoh Khp Titration Lab Report in this laboratory exercise you will carry out such a titration to standardize (determine the exact. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. You will precisely measure the concentration. \[\ce{c8h5ko4 (aq) + naoh (aq) → h2o + c8h4nako4 (aq)}\nonumber\] the. Standardize naoh/determine impure khp purpose:. Naoh Khp Titration Lab Report.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Naoh Khp Titration Lab Report — attach the graph to your lab report. Standardize naoh/determine impure khp purpose: a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. Determine the equivalence point (in ml of naoh) of this ph titration. The solution is then standardized, that is, its. khp works as a. Naoh Khp Titration Lab Report.
From www.chegg.com
Solved KHP Titration Report Sheet Concentration of NaOH Naoh Khp Titration Lab Report Sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. in this laboratory exercise you will carry out such a titration to standardize (determine the exact. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. The solution is then standardized,. Naoh Khp Titration Lab Report.
From www.numerade.com
SOLVED Name Loxe Sco Lab Section Dale REPORT ON EXPERIMENT 8 Naoh Khp Titration Lab Report — the titration reaction of khp with naoh is as follows: Sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. The same reasons we choose khp as our. khp works as a great primary standard for determination of naoh concentrations. in this laboratory exercise you will carry out such. Naoh Khp Titration Lab Report.
From www.numerade.com
SOLVED REPORT SHEET EXPERIMENT Titration of Acids and Bases 20 Naoh Khp Titration Lab Report The solution is then standardized, that is, its. You will precisely measure the concentration. — the titration reaction of khp with naoh is as follows: \[\ce{c8h5ko4 (aq) + naoh (aq) → h2o + c8h4nako4 (aq)}\nonumber\] the. Determine the equivalence point (in ml of naoh) of this ph titration. Sodium hydroxide solution of about 0.2 m is prepared in order. Naoh Khp Titration Lab Report.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Naoh Khp Titration Lab Report Standardize naoh/determine impure khp purpose: — attach the graph to your lab report. khp works as a great primary standard for determination of naoh concentrations. \[\ce{c8h5ko4 (aq) + naoh (aq) → h2o + c8h4nako4 (aq)}\nonumber\] the. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. You. Naoh Khp Titration Lab Report.
From studylib.net
CHM 115 Lab 3 Titration Standardize NaOH/Determine impure KHP Naoh Khp Titration Lab Report chm 115 lab 3 titration: The same reasons we choose khp as our. The solution is then standardized, that is, its. — the titration reaction of khp with naoh is as follows: a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. Standardize naoh/determine impure khp purpose:. Naoh Khp Titration Lab Report.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT Titration Of Acids And Bas... Naoh Khp Titration Lab Report a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. The solution is then standardized, that is, its. — the titration reaction of khp with naoh is as follows: chm 115 lab 3 titration: \[\ce{c8h5ko4 (aq) + naoh (aq) → h2o + c8h4nako4 (aq)}\nonumber\] the. —. Naoh Khp Titration Lab Report.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Naoh Khp Titration Lab Report The solution is then standardized, that is, its. chm 115 lab 3 titration: khp works as a great primary standard for determination of naoh concentrations. \[\ce{c8h5ko4 (aq) + naoh (aq) → h2o + c8h4nako4 (aq)}\nonumber\] the. Determine the equivalence point (in ml of naoh) of this ph titration. The same reasons we choose khp as our. —. Naoh Khp Titration Lab Report.
From www.numerade.com
SOLVED Part 1 KHP Titrations Question 2 (1 point) A student Naoh Khp Titration Lab Report chm 115 lab 3 titration: — attach the graph to your lab report. Sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. — the titration reaction of khp with naoh is as follows: You will precisely measure the concentration. Determine the equivalence point (in ml of naoh) of this. Naoh Khp Titration Lab Report.
From www.vrogue.co
Standardization Of Naoh With A Khp Solution Acid Base vrogue.co Naoh Khp Titration Lab Report \[\ce{c8h5ko4 (aq) + naoh (aq) → h2o + c8h4nako4 (aq)}\nonumber\] the. Determine the equivalence point (in ml of naoh) of this ph titration. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. The solution is then standardized, that is, its. The same reasons we choose khp as our.. Naoh Khp Titration Lab Report.
From www.numerade.com
SOLVED Experiment 1 AcidBase Titration Standardization of NaOH Naoh Khp Titration Lab Report chm 115 lab 3 titration: \[\ce{c8h5ko4 (aq) + naoh (aq) → h2o + c8h4nako4 (aq)}\nonumber\] the. khp works as a great primary standard for determination of naoh concentrations. in this laboratory exercise you will carry out such a titration to standardize (determine the exact. The same reasons we choose khp as our. Determine the equivalence point (in. Naoh Khp Titration Lab Report.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Naoh Khp Titration Lab Report — attach the graph to your lab report. in this laboratory exercise you will carry out such a titration to standardize (determine the exact. Determine the equivalence point (in ml of naoh) of this ph titration. Standardize naoh/determine impure khp purpose: \[\ce{c8h5ko4 (aq) + naoh (aq) → h2o + c8h4nako4 (aq)}\nonumber\] the. The same reasons we choose khp. Naoh Khp Titration Lab Report.
From exocnnftw.blob.core.windows.net
How Do You Calculate The Concentration Of Naoh In A Titration at Naoh Khp Titration Lab Report You will precisely measure the concentration. Standardize naoh/determine impure khp purpose: in this laboratory exercise you will carry out such a titration to standardize (determine the exact. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. Sodium hydroxide solution of about 0.2 m is prepared in order. Naoh Khp Titration Lab Report.
From www.numerade.com
SOLVED Question 3 Please Experiment 5 AcidBase Titration Naoh Khp Titration Lab Report You will precisely measure the concentration. The solution is then standardized, that is, its. chm 115 lab 3 titration: Sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. Standardize naoh/determine impure khp purpose: — attach the graph to your lab report. in this laboratory exercise you will carry out. Naoh Khp Titration Lab Report.
From studybreathings.z21.web.core.windows.net
How To Standardize Naoh Naoh Khp Titration Lab Report khp works as a great primary standard for determination of naoh concentrations. a titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples. — attach the graph to your lab report. The solution is then standardized, that is, its. \[\ce{c8h5ko4 (aq) + naoh (aq) → h2o + c8h4nako4. Naoh Khp Titration Lab Report.
From www.chegg.com
Solved KHP Titration Report Sheet Concentration of NaOH Naoh Khp Titration Lab Report chm 115 lab 3 titration: You will precisely measure the concentration. Determine the equivalence point (in ml of naoh) of this ph titration. khp works as a great primary standard for determination of naoh concentrations. Sodium hydroxide solution of about 0.2 m is prepared in order to be used in exp 12b. Standardize naoh/determine impure khp purpose: . Naoh Khp Titration Lab Report.