V K H Where K Is A Constant . simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. Where k is a constant. the proportionality constant, r, is called the gas constant and has the value 0.08206 (l•atm)/(k•mol), 8.3145 j/(k•mol), or. V is inversely proportional to h. (iii) find the set of values of t for which the acceleration of p is positive. Because v = k h v=\frac. V v is inversely proportional to h h. the ratio of volume to temperature is constant when pressure is constant. charles’s law states that when pressure is constant, the volume is directly proportional to the temperature when containing a fixed amount. V v is directly proportional to \frac {1} {h} h 1. This relationship is known as charles' law or. V is directly proportional to 1 7 \frac {1}{7} 7 1. v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14 ≤ t ≤ 20.
from www.youtube.com
charles’s law states that when pressure is constant, the volume is directly proportional to the temperature when containing a fixed amount. the proportionality constant, r, is called the gas constant and has the value 0.08206 (l•atm)/(k•mol), 8.3145 j/(k•mol), or. (iii) find the set of values of t for which the acceleration of p is positive. Where k is a constant. V v is inversely proportional to h h. V is directly proportional to 1 7 \frac {1}{7} 7 1. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. This relationship is known as charles' law or. v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14 ≤ t ≤ 20. the ratio of volume to temperature is constant when pressure is constant.
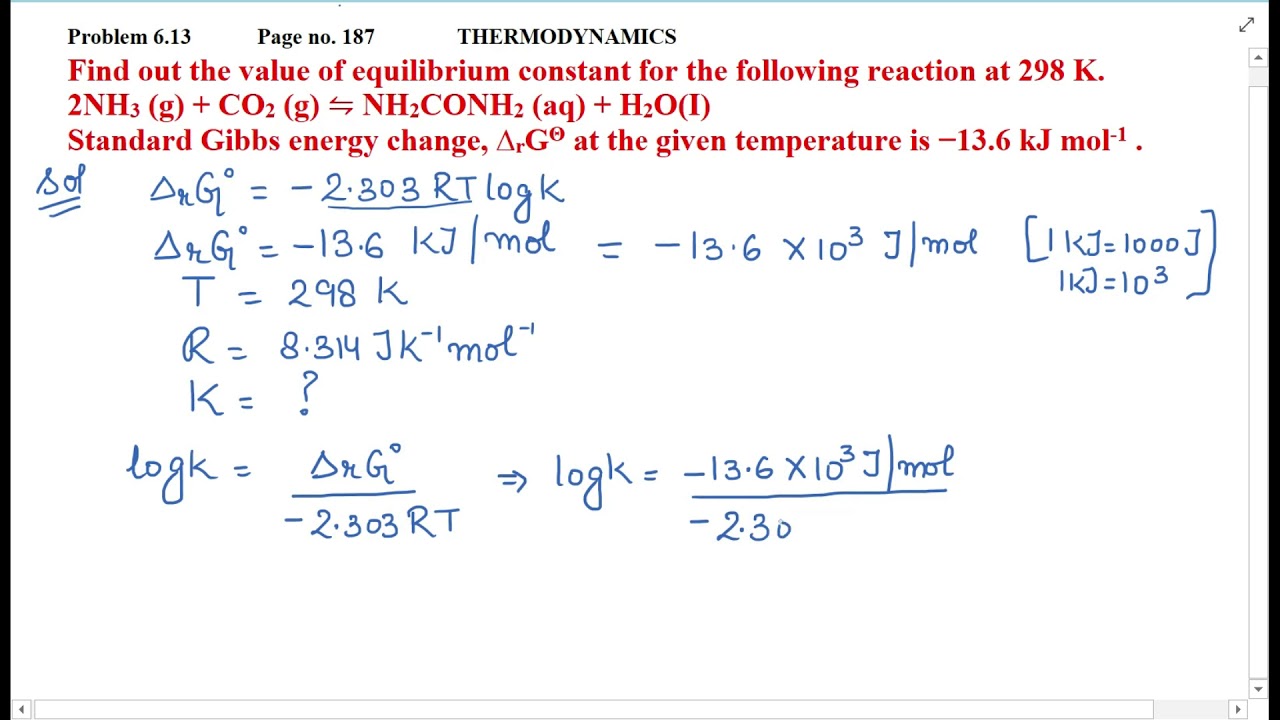
Find out the value of equilibrium constant for the following reaction
V K H Where K Is A Constant (iii) find the set of values of t for which the acceleration of p is positive. (iii) find the set of values of t for which the acceleration of p is positive. This relationship is known as charles' law or. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. V is directly proportional to 1 7 \frac {1}{7} 7 1. Because v = k h v=\frac. the ratio of volume to temperature is constant when pressure is constant. V v is inversely proportional to h h. V is inversely proportional to h. v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14 ≤ t ≤ 20. the proportionality constant, r, is called the gas constant and has the value 0.08206 (l•atm)/(k•mol), 8.3145 j/(k•mol), or. V v is directly proportional to \frac {1} {h} h 1. Where k is a constant. charles’s law states that when pressure is constant, the volume is directly proportional to the temperature when containing a fixed amount.
From byjus.com
A particle is moving with v= k( y i cap + x jcap) where k is constant V K H Where K Is A Constant This relationship is known as charles' law or. (iii) find the set of values of t for which the acceleration of p is positive. V is directly proportional to 1 7 \frac {1}{7} 7 1. charles’s law states that when pressure is constant, the volume is directly proportional to the temperature when containing a fixed amount. Where k is. V K H Where K Is A Constant.
From askfilo.com
Blectric potential at a point is v=pπ where K ' is constant; Electric fi.. V K H Where K Is A Constant V v is inversely proportional to h h. v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14 ≤ t ≤ 20. V v is directly proportional to \frac {1} {h} h 1. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. This relationship is known as. V K H Where K Is A Constant.
From www.numerade.com
SOLVED Ohm's law V = IR describes the relationship between the voltage V K H Where K Is A Constant simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. V v is inversely proportional to h h. V is directly proportional to 1 7 \frac {1}{7} 7 1. the proportionality constant, r, is called the gas constant and has the value 0.08206 (l•atm)/(k•mol), 8.3145 j/(k•mol), or. This relationship is known as charles' law. V K H Where K Is A Constant.
From www.coursehero.com
[Solved] Find the specific heat at constant pressure of nitrogen gas V K H Where K Is A Constant Where k is a constant. charles’s law states that when pressure is constant, the volume is directly proportional to the temperature when containing a fixed amount. V is directly proportional to 1 7 \frac {1}{7} 7 1. V v is directly proportional to \frac {1} {h} h 1. simply put, boyle's states that for a gas at constant. V K H Where K Is A Constant.
From www.youtube.com
Find the Value of k in Quadratic Equations when One Root is Given V K H Where K Is A Constant V is inversely proportional to h. charles’s law states that when pressure is constant, the volume is directly proportional to the temperature when containing a fixed amount. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14. V K H Where K Is A Constant.
From scoop.eduncle.com
The intemal energy of an ideal gas follows the equation u= 3.5 pv+ k V K H Where K Is A Constant V v is directly proportional to \frac {1} {h} h 1. Because v = k h v=\frac. v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14 ≤ t ≤ 20. the proportionality constant, r, is called the gas constant and has the value 0.08206 (l•atm)/(k•mol), 8.3145 j/(k•mol), or. V is directly. V K H Where K Is A Constant.
From www.toppr.com
A particle moves uniformly with a speed of v along a parabolic path y V K H Where K Is A Constant simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. Where k is a constant. Because v = k h v=\frac. V v is inversely proportional to h h. the proportionality constant, r, is called the gas constant and has the value 0.08206 (l•atm)/(k•mol), 8.3145 j/(k•mol), or. V is inversely proportional to h. This. V K H Where K Is A Constant.
From askfilo.com
A force F=−k(yi^+xj^ ) (where k is constant) acts Filo V K H Where K Is A Constant the ratio of volume to temperature is constant when pressure is constant. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. V is inversely proportional to h. V v is directly proportional to \frac {1} {h} h 1. (iii) find the set of values of t for which the acceleration of p is. V K H Where K Is A Constant.
From www.numerade.com
SOLVED The velocity of a particle traveling along a straight line is v V K H Where K Is A Constant charles’s law states that when pressure is constant, the volume is directly proportional to the temperature when containing a fixed amount. v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14 ≤ t ≤ 20. V is inversely proportional to h. the ratio of volume to temperature is constant when pressure. V K H Where K Is A Constant.
From www.toppr.com
Force acting on a particle moving in a straight line varies with the V K H Where K Is A Constant V v is inversely proportional to h h. Because v = k h v=\frac. the ratio of volume to temperature is constant when pressure is constant. This relationship is known as charles' law or. V v is directly proportional to \frac {1} {h} h 1. Where k is a constant. simply put, boyle's states that for a gas. V K H Where K Is A Constant.
From www.toppr.com
Let f and g be differentiable on [0 , 1] such that f(0) = 2 , g(0) = 0 V K H Where K Is A Constant This relationship is known as charles' law or. the ratio of volume to temperature is constant when pressure is constant. charles’s law states that when pressure is constant, the volume is directly proportional to the temperature when containing a fixed amount. V is directly proportional to 1 7 \frac {1}{7} 7 1. v = k for 4. V K H Where K Is A Constant.
From www.numerade.com
SOLVEDThe ?alpor pressures of salid and liquid bydrogen iodide cun be V K H Where K Is A Constant Because v = k h v=\frac. This relationship is known as charles' law or. V v is directly proportional to \frac {1} {h} h 1. (iii) find the set of values of t for which the acceleration of p is positive. the ratio of volume to temperature is constant when pressure is constant. v = k for 4. V K H Where K Is A Constant.
From byjus.com
What is the relation between Kh ( Henry's law constant) and molecular V K H Where K Is A Constant v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14 ≤ t ≤ 20. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. (iii) find the set of values of t for which the acceleration of p is positive. the ratio of volume to temperature is. V K H Where K Is A Constant.
From www.chegg.com
Solved Consider the 2d harmonic oscillator with H = V K H Where K Is A Constant the proportionality constant, r, is called the gas constant and has the value 0.08206 (l•atm)/(k•mol), 8.3145 j/(k•mol), or. the ratio of volume to temperature is constant when pressure is constant. (iii) find the set of values of t for which the acceleration of p is positive. V v is inversely proportional to h h. simply put, boyle's. V K H Where K Is A Constant.
From studylibplimsoll.z21.web.core.windows.net
What Is Constant Rate V K H Where K Is A Constant the proportionality constant, r, is called the gas constant and has the value 0.08206 (l•atm)/(k•mol), 8.3145 j/(k•mol), or. V is inversely proportional to h. This relationship is known as charles' law or. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. V v is directly proportional to \frac {1} {h} h 1. V. V K H Where K Is A Constant.
From www.toppr.com
A particle of mass m is free to move along the x axis and has V K H Where K Is A Constant Where k is a constant. the ratio of volume to temperature is constant when pressure is constant. v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14 ≤ t ≤ 20. V v is directly proportional to \frac {1} {h} h 1. (iii) find the set of values of t for which. V K H Where K Is A Constant.
From www.numerade.com
SOLVED Population Model Another population model is given by dP/dt V K H Where K Is A Constant V v is directly proportional to \frac {1} {h} h 1. Where k is a constant. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. charles’s law states that when pressure is constant, the volume is directly proportional to the temperature when containing a fixed amount. the proportionality constant, r, is called. V K H Where K Is A Constant.
From www.toppr.com
A force vec F = K (yvec i + xvec j ) , where k is a positive constant V K H Where K Is A Constant the proportionality constant, r, is called the gas constant and has the value 0.08206 (l•atm)/(k•mol), 8.3145 j/(k•mol), or. V v is directly proportional to \frac {1} {h} h 1. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. v = k for 4 ≤ t ≤ 14, v = 68 − 2t. V K H Where K Is A Constant.
From www.gauthmath.com
Solved 4) The volume (V) of a cone varies jointly as its height (h V K H Where K Is A Constant V v is inversely proportional to h h. This relationship is known as charles' law or. the proportionality constant, r, is called the gas constant and has the value 0.08206 (l•atm)/(k•mol), 8.3145 j/(k•mol), or. Where k is a constant. (iii) find the set of values of t for which the acceleration of p is positive. simply put, boyle's. V K H Where K Is A Constant.
From www.chegg.com
Solved 1 ƏF, = Problem 1 Suppose the electric field in some V K H Where K Is A Constant v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14 ≤ t ≤ 20. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. V is inversely proportional to h. charles’s law states that when pressure is constant, the volume is directly proportional to the temperature when. V K H Where K Is A Constant.
From www.youtube.com
Find out the value of equilibrium constant for the following reaction V K H Where K Is A Constant Where k is a constant. v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14 ≤ t ≤ 20. the proportionality constant, r, is called the gas constant and has the value 0.08206 (l•atm)/(k•mol), 8.3145 j/(k•mol), or. V is directly proportional to 1 7 \frac {1}{7} 7 1. Because v = k. V K H Where K Is A Constant.
From www.toppr.com
A particle is moving with velocity vec v = k(yvec i + xvec j) where k V K H Where K Is A Constant (iii) find the set of values of t for which the acceleration of p is positive. v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14 ≤ t ≤ 20. Where k is a constant. Because v = k h v=\frac. charles’s law states that when pressure is constant, the volume is. V K H Where K Is A Constant.
From www.chegg.com
Solved When a certain polyatomic gas undergoes adiabatic V K H Where K Is A Constant the proportionality constant, r, is called the gas constant and has the value 0.08206 (l•atm)/(k•mol), 8.3145 j/(k•mol), or. This relationship is known as charles' law or. (iii) find the set of values of t for which the acceleration of p is positive. the ratio of volume to temperature is constant when pressure is constant. V v is directly. V K H Where K Is A Constant.
From dxoftwylg.blob.core.windows.net
Ideal Gas Graph at Catherine Rooker blog V K H Where K Is A Constant V is directly proportional to 1 7 \frac {1}{7} 7 1. Where k is a constant. the ratio of volume to temperature is constant when pressure is constant. V v is directly proportional to \frac {1} {h} h 1. V v is inversely proportional to h h. v = k for 4 ≤ t ≤ 14, v =. V K H Where K Is A Constant.
From www.numerade.com
SOLVED The acceleration of a particle is defined by the relation a V K H Where K Is A Constant This relationship is known as charles' law or. V v is inversely proportional to h h. Because v = k h v=\frac. the ratio of volume to temperature is constant when pressure is constant. V is inversely proportional to h. V is directly proportional to 1 7 \frac {1}{7} 7 1. v = k for 4 ≤ t. V K H Where K Is A Constant.
From askfilo.com
A spring gun having a spring constant k is placed at a height h. Aball of.. V K H Where K Is A Constant V v is inversely proportional to h h. V is directly proportional to 1 7 \frac {1}{7} 7 1. This relationship is known as charles' law or. V v is directly proportional to \frac {1} {h} h 1. the ratio of volume to temperature is constant when pressure is constant. (iii) find the set of values of t for. V K H Where K Is A Constant.
From www.masterorganicchemistry.com
Equilibrium and Energy Relationships Master Organic Chemistry V K H Where K Is A Constant Where k is a constant. V v is inversely proportional to h h. Because v = k h v=\frac. V is directly proportional to 1 7 \frac {1}{7} 7 1. This relationship is known as charles' law or. (iii) find the set of values of t for which the acceleration of p is positive. V is inversely proportional to h.. V K H Where K Is A Constant.
From www.doubtnut.com
The graph between 1//lambda and stopping potential (V) of three metals V K H Where K Is A Constant the proportionality constant, r, is called the gas constant and has the value 0.08206 (l•atm)/(k•mol), 8.3145 j/(k•mol), or. the ratio of volume to temperature is constant when pressure is constant. Because v = k h v=\frac. V v is inversely proportional to h h. charles’s law states that when pressure is constant, the volume is directly proportional. V K H Where K Is A Constant.
From edurev.in
A force f= k(yixj) (where k is constant) act on a particle moving in V K H Where K Is A Constant V v is directly proportional to \frac {1} {h} h 1. V v is inversely proportional to h h. Where k is a constant. This relationship is known as charles' law or. V is directly proportional to 1 7 \frac {1}{7} 7 1. (iii) find the set of values of t for which the acceleration of p is positive. . V K H Where K Is A Constant.
From www.chegg.com
Solved Let h(x)=e−x+kx, where k is any constant. (a) Find V K H Where K Is A Constant V is inversely proportional to h. the ratio of volume to temperature is constant when pressure is constant. V v is inversely proportional to h h. v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14 ≤ t ≤ 20. This relationship is known as charles' law or. simply put, boyle's. V K H Where K Is A Constant.
From www.chegg.com
Solved Considering the following RL series circuit Use 3.142 V K H Where K Is A Constant (iii) find the set of values of t for which the acceleration of p is positive. simply put, boyle's states that for a gas at constant temperature, pressure multiplied by. V v is inversely proportional to h h. the ratio of volume to temperature is constant when pressure is constant. Where k is a constant. V v is. V K H Where K Is A Constant.
From www.chegg.com
Solved 6 a What current density would produce the vector V K H Where K Is A Constant charles’s law states that when pressure is constant, the volume is directly proportional to the temperature when containing a fixed amount. v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14 ≤ t ≤ 20. V v is inversely proportional to h h. V is inversely proportional to h. Because v =. V K H Where K Is A Constant.
From www.gauthmath.com
Solved 1 V= k/H where k is a constant. Which two statements are V K H Where K Is A Constant Where k is a constant. the ratio of volume to temperature is constant when pressure is constant. charles’s law states that when pressure is constant, the volume is directly proportional to the temperature when containing a fixed amount. Because v = k h v=\frac. V v is directly proportional to \frac {1} {h} h 1. v =. V K H Where K Is A Constant.
From www.toppr.com
A ring of circular cross section is made of a material of restrivity V K H Where K Is A Constant v = k for 4 ≤ t ≤ 14, v = 68 − 2t for 14 ≤ t ≤ 20. charles’s law states that when pressure is constant, the volume is directly proportional to the temperature when containing a fixed amount. the ratio of volume to temperature is constant when pressure is constant. Where k is a. V K H Where K Is A Constant.
From www.toppr.com
14. A particle is moving with a velocity v=k(yî + xj), where k is a V K H Where K Is A Constant Because v = k h v=\frac. This relationship is known as charles' law or. charles’s law states that when pressure is constant, the volume is directly proportional to the temperature when containing a fixed amount. V v is inversely proportional to h h. the proportionality constant, r, is called the gas constant and has the value 0.08206 (l•atm)/(k•mol),. V K H Where K Is A Constant.