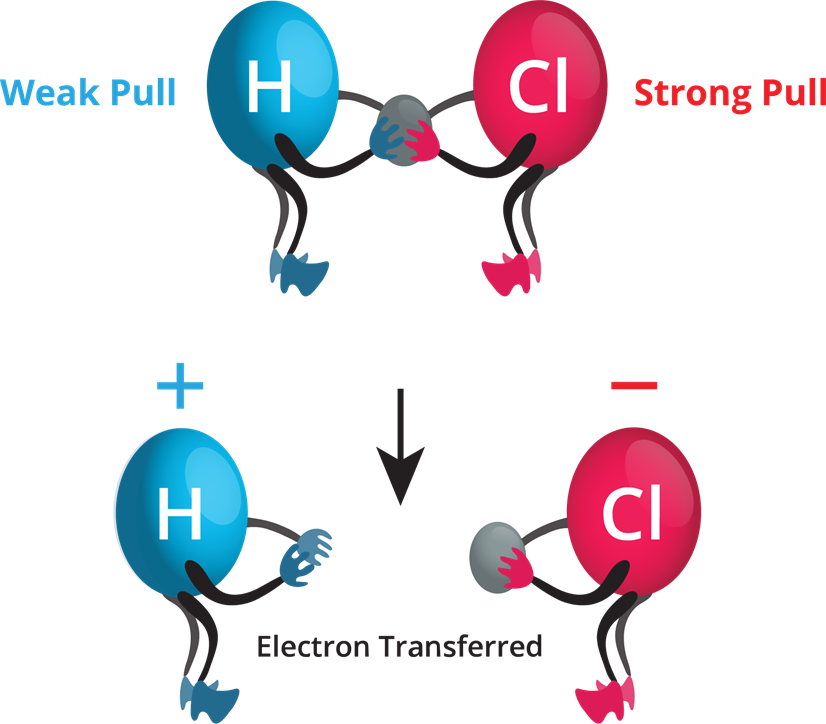
Chlorine Gas Electronegativity Difference . This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine to form na Think of sodium chloride as if. It looks at the way that electronegativity. In nacl, δχ is 2.23. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). You can also use our tool as an. You can see, for instance, in period 3 that sodium (na) has an electronegativity calculation of 0.93, while chlorine (cl), the last element in that period, has an electronegativity of 3.16. Cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; Hence the two chlorine atoms share the bonding electrons equally. It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. Why does electronegativity increase across a period? The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. This page explains what electronegativity is, and how and why it varies around the periodic table. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon).
from www.yaclass.in
In nacl, δχ is 2.23. This page explains what electronegativity is, and how and why it varies around the periodic table. Cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine to form na Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). Think of sodium chloride as if. It looks at the way that electronegativity. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values.
Electronegativity — lesson. Science State Board, Class 10.
Chlorine Gas Electronegativity Difference The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. It looks at the way that electronegativity. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine to form na This page explains what electronegativity is, and how and why it varies around the periodic table. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. Why does electronegativity increase across a period? You can see, for instance, in period 3 that sodium (na) has an electronegativity calculation of 0.93, while chlorine (cl), the last element in that period, has an electronegativity of 3.16. In nacl, δχ is 2.23. It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. You can also use our tool as an. Cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; Hence the two chlorine atoms share the bonding electrons equally. Think of sodium chloride as if.
From slideplayer.com
State University of New York at Brockport ppt download Chlorine Gas Electronegativity Difference You can also use our tool as an. Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. Cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; Think of sodium chloride as if. This page explains what electronegativity is, and how and why it varies around the periodic table. Consider. Chlorine Gas Electronegativity Difference.
From tr.pinterest.com
Illustration about Periodic table of elements with electronegativity Chlorine Gas Electronegativity Difference Cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. In nacl, δχ is 2.23. This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to. Chlorine Gas Electronegativity Difference.
From www.periodic-table.org
Chlorine Electronegativity Cl Chlorine Gas Electronegativity Difference This page explains what electronegativity is, and how and why it varies around the periodic table. Cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; You can see, for instance, in period 3 that sodium (na) has an electronegativity calculation of 0.93, while chlorine (cl), the last element in that period, has an electronegativity of 3.16. In. Chlorine Gas Electronegativity Difference.
From iperiodictable.com
Periodic Table Electronegativity Periodic Table Chlorine Gas Electronegativity Difference In nacl, δχ is 2.23. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). Hence the two chlorine atoms share the bonding electrons equally. Consider sodium at the. Chlorine Gas Electronegativity Difference.
From mungfali.com
Electronegativity Difference Chart Chlorine Gas Electronegativity Difference It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine to form na You can see, for instance, in period 3 that sodium (na) has an. Chlorine Gas Electronegativity Difference.
From www.nanowerk.com
Electronegativity explained Chlorine Gas Electronegativity Difference Why does electronegativity increase across a period? It looks at the way that electronegativity. In nacl, δχ is 2.23. You can see, for instance, in period 3 that sodium (na) has an electronegativity calculation of 0.93, while chlorine (cl), the last element in that period, has an electronegativity of 3.16. You can also use our tool as an. The electronegativity. Chlorine Gas Electronegativity Difference.
From www.numerade.com
SOLVED Need help. What is Electronegativity difference? Thank you Chlorine Gas Electronegativity Difference This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine to form na Think of sodium chloride as if. You can also use our tool as an. It looks at the way that electronegativity. It is caused by the attractive electrostatic force between the. Chlorine Gas Electronegativity Difference.
From www.geeksforgeeks.org
Electronegativity Definition, Meaning, Periodic Trends, Examples Chlorine Gas Electronegativity Difference This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine to form na Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). You can see, for instance, in period 3 that sodium (na) has an. Chlorine Gas Electronegativity Difference.
From dona.tompkinscountystructuralracism.org
Electronegativity Values Chart Understanding The Chemistry Of Elements Chlorine Gas Electronegativity Difference It looks at the way that electronegativity. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). You can see, for instance, in period 3 that sodium (na) has an electronegativity calculation of 0.93, while chlorine (cl), the last element in that period, has an electronegativity of 3.16. It is caused by. Chlorine Gas Electronegativity Difference.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine Chlorine Gas Electronegativity Difference Think of sodium chloride as if. You can see, for instance, in period 3 that sodium (na) has an electronegativity calculation of 0.93, while chlorine (cl), the last element in that period, has an electronegativity of 3.16. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). It. Chlorine Gas Electronegativity Difference.
From slideplayer.com
CHEMICAL BONDING. ppt download Chlorine Gas Electronegativity Difference Cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; In nacl, δχ is 2.23. This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine to form na You can see, for instance, in period 3 that sodium (na) has an electronegativity. Chlorine Gas Electronegativity Difference.
From mavink.com
Electronegativity Difference Chart Chlorine Gas Electronegativity Difference The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). It looks at the way that electronegativity. Why does electronegativity increase across a period? This page explains what electronegativity is, and how and why it varies around the periodic table. This high value is typical of an ionic. Chlorine Gas Electronegativity Difference.
From www.dreamstime.com
Chlorine Chemical Element with 17 Atomic Number, Atomic Mass and Chlorine Gas Electronegativity Difference The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. It looks at the way that electronegativity. In nacl, δχ is 2.23. You can also use our tool as an.. Chlorine Gas Electronegativity Difference.
From narodnatribuna.info
Electronegativity Definition And Examples Chlorine Gas Electronegativity Difference You can see, for instance, in period 3 that sodium (na) has an electronegativity calculation of 0.93, while chlorine (cl), the last element in that period, has an electronegativity of 3.16. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Cl 2 must be nonpolar because the electronegativity difference (δχ). Chlorine Gas Electronegativity Difference.
From www.slideserve.com
PPT Electronegativity and Polarity PowerPoint Presentation, free Chlorine Gas Electronegativity Difference This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine to form na The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). Hence the two chlorine atoms share the bonding electrons. Chlorine Gas Electronegativity Difference.
From periodictable.me
How to Calculate Electronegativity of Element Chlorine Gas Electronegativity Difference Hence the two chlorine atoms share the bonding electrons equally. Think of sodium chloride as if. This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine to form na This page explains what electronegativity is, and how and why it varies around the periodic. Chlorine Gas Electronegativity Difference.
From www.dreamstime.com
Chlorine Chemical Element with First Ionization Energy, Atomic Mass and Chlorine Gas Electronegativity Difference Hence the two chlorine atoms share the bonding electrons equally. This page explains what electronegativity is, and how and why it varies around the periodic table. Why does electronegativity increase across a period? Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. You can see, for instance, in period 3 that sodium. Chlorine Gas Electronegativity Difference.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Chlorine Gas Electronegativity Difference In nacl, δχ is 2.23. Think of sodium chloride as if. This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine to form na The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Chlorine Gas Electronegativity Difference.
From www.wikihow.com
3 Clear and Easy Ways to Calculate Electronegativity wikiHow Chlorine Gas Electronegativity Difference Why does electronegativity increase across a period? You can also use our tool as an. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine. Chlorine Gas Electronegativity Difference.
From slideplayer.com
State University of New York at Brockport ppt download Chlorine Gas Electronegativity Difference This page explains what electronegativity is, and how and why it varies around the periodic table. Why does electronegativity increase across a period? Think of sodium chloride as if. Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means. Chlorine Gas Electronegativity Difference.
From sciencenotes.org
List of Electronegativity Values of the Elements Chlorine Gas Electronegativity Difference The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). Why does electronegativity increase across a period? In nacl, δχ is 2.23. This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine. Chlorine Gas Electronegativity Difference.
From chemdictionary.org
Electronegativity Definition And Examples Chemistry Dictionary Chlorine Gas Electronegativity Difference You can also use our tool as an. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). This page explains what electronegativity is, and how and why it varies around the periodic table. It looks at the way that electronegativity. Why does electronegativity increase across a period? You can see, for. Chlorine Gas Electronegativity Difference.
From www.pinterest.com
Electronegativity Definition and Trend Chlorine Gas Electronegativity Difference Cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble gas, argon). Think of sodium chloride as if. Hence the two chlorine atoms share the bonding electrons equally. Why does electronegativity increase across a period? It looks at the way that electronegativity.. Chlorine Gas Electronegativity Difference.
From mungfali.com
Electronegativity Trend Explained Chlorine Gas Electronegativity Difference It looks at the way that electronegativity. This page explains what electronegativity is, and how and why it varies around the periodic table. Why does electronegativity increase across a period? You can see, for instance, in period 3 that sodium (na) has an electronegativity calculation of 0.93, while chlorine (cl), the last element in that period, has an electronegativity of. Chlorine Gas Electronegativity Difference.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Chlorine Gas Electronegativity Difference Think of sodium chloride as if. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). Cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; You can also use our tool as an. Hence the two chlorine atoms share the bonding electrons equally. This page. Chlorine Gas Electronegativity Difference.
From ncvs3.books.nba.co.za
Unit 2 Electronegativity and polarity National Curriculum Chlorine Gas Electronegativity Difference It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. You can also use our tool as an. Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. Think of sodium chloride as if. The electronegativity calculator allows you to calculate the type of bond. Chlorine Gas Electronegativity Difference.
From www.yaclass.in
Electronegativity — lesson. Science State Board, Class 10. Chlorine Gas Electronegativity Difference You can see, for instance, in period 3 that sodium (na) has an electronegativity calculation of 0.93, while chlorine (cl), the last element in that period, has an electronegativity of 3.16. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). Hence the two chlorine atoms share the. Chlorine Gas Electronegativity Difference.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Chlorine Gas Electronegativity Difference Hence the two chlorine atoms share the bonding electrons equally. This page explains what electronegativity is, and how and why it varies around the periodic table. You can see, for instance, in period 3 that sodium (na) has an electronegativity calculation of 0.93, while chlorine (cl), the last element in that period, has an electronegativity of 3.16. The electronegativity calculator. Chlorine Gas Electronegativity Difference.
From www.bigstockphoto.com
Electronegativity Image & Photo (Free Trial) Bigstock Chlorine Gas Electronegativity Difference It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. You can also use our tool as an. This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine to form na This page explains what electronegativity. Chlorine Gas Electronegativity Difference.
From fr.dreamstime.com
Table Périodique D'Electronegativity Illustration Stock Illustration Chlorine Gas Electronegativity Difference The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). Cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; Hence the two chlorine atoms share the bonding electrons equally. This page explains what electronegativity is, and how and why it varies around the periodic table.. Chlorine Gas Electronegativity Difference.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Chlorine Gas Electronegativity Difference Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. This page explains what electronegativity is, and how and why it varies around the periodic table. It looks at the way that electronegativity. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. You. Chlorine Gas Electronegativity Difference.
From mavink.com
Periodic Table With Electronegativity Values Chlorine Gas Electronegativity Difference Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. It looks at the way that electronegativity. Why does electronegativity increase across a period? This page explains what electronegativity is, and how and why it varies around the periodic table. Cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; The. Chlorine Gas Electronegativity Difference.
From mungfali.com
Electronegativity Chart Printable Chlorine Gas Electronegativity Difference You can also use our tool as an. Cl 2 must be nonpolar because the electronegativity difference (δχ) is zero; Think of sodium chloride as if. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). Hence the two chlorine atoms share the bonding electrons equally. It is. Chlorine Gas Electronegativity Difference.
From www.slideserve.com
PPT Covalent Bonds Electronegativity differences and ionic/polar Chlorine Gas Electronegativity Difference You can also use our tool as an. This page explains what electronegativity is, and how and why it varies around the periodic table. This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine to form na In nacl, δχ is 2.23. You can. Chlorine Gas Electronegativity Difference.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Chlorine Gas Electronegativity Difference In nacl, δχ is 2.23. This page explains what electronegativity is, and how and why it varies around the periodic table. This high value is typical of an ionic compound (δχ ≥ ≈1.5) and means that the valence electron of sodium has been completely transferred to chlorine to form na Consider sodium at the beginning of period 3 and chlorine. Chlorine Gas Electronegativity Difference.