Absorption In Chemistry . Absorption differs from from adsorption, since the. A gas absorbed by a liquid. In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state (e.g.,. Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). Absorption — one substance enters the bulk, or volume, of another substance e.g. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (\(\lambda\)) by a sample, and can be used to generate a spectrum, which is a plot of the absorbance as a function of the wavelength. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. Adsorption — takes place on the surface of a substrate. Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy.
from mavink.com
Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). Absorption differs from from adsorption, since the. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. Absorption — one substance enters the bulk, or volume, of another substance e.g. In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state (e.g.,. A gas absorbed by a liquid. Adsorption — takes place on the surface of a substrate. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (\(\lambda\)) by a sample, and can be used to generate a spectrum, which is a plot of the absorbance as a function of the wavelength.
Absorption Spectrum Of Hydrogen
Absorption In Chemistry Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. Adsorption — takes place on the surface of a substrate. Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). Absorption differs from from adsorption, since the. A gas absorbed by a liquid. In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state (e.g.,. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (\(\lambda\)) by a sample, and can be used to generate a spectrum, which is a plot of the absorbance as a function of the wavelength. Absorption — one substance enters the bulk, or volume, of another substance e.g. Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy.
From www.slideserve.com
PPT Light absorption PowerPoint Presentation, free download ID2699464 Absorption In Chemistry Absorption differs from from adsorption, since the. Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. Absorption — one substance enters the bulk, or volume, of another substance e.g. In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state. Absorption In Chemistry.
From www.youtube.com
A.6.2 Describe the principles of atomic absorption IB Chemistry SL Absorption In Chemistry A gas absorbed by a liquid. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. Absorption — one substance enters the bulk, or volume, of another substance e.g. In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a. Absorption In Chemistry.
From en.wikipedia.org
Absorption (chemistry) Wikipedia Absorption In Chemistry In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state (e.g.,. Absorption — one substance enters the bulk, or volume, of another substance e.g. Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). The major difference between adsorption and. Absorption In Chemistry.
From present5.com
CHAPTER 7 ABSORPTION 1 ABSORPTION GIT Absorption In Chemistry Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (\(\lambda\)) by a sample, and can be used to generate a spectrum, which is a plot of the absorbance as a function of the wavelength. Absorption —. Absorption In Chemistry.
From myilikeimages.blogspot.com
At The Equilibrium Position In The Process Of Adsorption Experiment Absorption In Chemistry Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). Absorption differs from from adsorption, since the. Adsorption — takes place on the surface of a substrate. Absorption — one substance enters the bulk, or volume, of another substance e.g. In chemistry, absorption is a process by which a substance incorporated in one. Absorption In Chemistry.
From learn.careers360.com
Surface Chemistry Definition, Colloid Formation, Catalysis, Notes and Absorption In Chemistry Absorption — one substance enters the bulk, or volume, of another substance e.g. In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state (e.g.,. Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. Absorption differs from from adsorption, since. Absorption In Chemistry.
From www.slideshare.net
IB Chemistry on Absorption Spectrum and Line Emission/Absorption Spec… Absorption In Chemistry Absorption differs from from adsorption, since the. Absorption — one substance enters the bulk, or volume, of another substance e.g. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (\(\lambda\)) by a sample, and can be used to generate a spectrum, which is a plot of the absorbance as a function of the. Absorption In Chemistry.
From www.slideserve.com
PPT Ch. 8 The Quantum Mechanical Atom PowerPoint Presentation, free Absorption In Chemistry Absorption differs from from adsorption, since the. Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). Adsorption — takes place on the surface of a substrate. Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. In chemistry, absorption is a process by which a. Absorption In Chemistry.
From www.youtube.com
C3 Absorption, Line, Emission and Continuous Spectra [SL IB Chemistry Absorption In Chemistry In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state (e.g.,. Absorption differs from from adsorption, since the. A gas absorbed by a liquid. Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. Absorption — one substance enters the. Absorption In Chemistry.
From www.slideserve.com
PPT Chapter 4 Electron Configurations and Quantum Chemistry Absorption In Chemistry Absorption differs from from adsorption, since the. A gas absorbed by a liquid. Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. A spectrophotometer in an instrument that measures the amount of light absorbed at. Absorption In Chemistry.
From www.toppr.com
Define emission spectron and absorption spectrum. Absorption In Chemistry Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. Absorption differs from from adsorption, since the. Absorption — one substance enters the bulk, or volume, of another substance e.g. In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state. Absorption In Chemistry.
From chem.libretexts.org
10.2 Spectroscopy Based on Absorption Chemistry LibreTexts Absorption In Chemistry Adsorption — takes place on the surface of a substrate. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. Absorption differs from from adsorption, since the. In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state. Absorption In Chemistry.
From www.researchgate.net
Chemical absorption process Download Scientific Diagram Absorption In Chemistry Absorption differs from from adsorption, since the. Adsorption — takes place on the surface of a substrate. A gas absorbed by a liquid. Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of. Absorption In Chemistry.
From www.youtube.com
What is Effect of Solvent on UV Absorption Spectra Spectroscopy Absorption In Chemistry A gas absorbed by a liquid. Absorption — one substance enters the bulk, or volume, of another substance e.g. Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. Absorption differs from from adsorption, since the. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (\(\lambda\)). Absorption In Chemistry.
From www.slideshare.net
IB Chemistry on Absorption Spectrum and Line Emission/Absorption Spec… Absorption In Chemistry Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (\(\lambda\)) by a sample, and can be used to generate a spectrum, which is a plot of the absorbance as a function of the wavelength. Adsorption —. Absorption In Chemistry.
From sites.middlebury.edu
Colors, Part I Absorption General Chemistry Lab News Absorption In Chemistry Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). A gas absorbed by a liquid. Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (\(\lambda\)) by a sample,. Absorption In Chemistry.
From present5.com
Principle of Atomic Absorption Spectrophotometry Mr Charnchai Suracheep Absorption In Chemistry A gas absorbed by a liquid. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (\(\lambda\)) by a sample, and can be used to generate a spectrum, which is a plot of the absorbance as a function of the wavelength. In chemistry, absorption is a process by which a substance incorporated in one. Absorption In Chemistry.
From chem.libretexts.org
14.1 Vocabulary Chemistry LibreTexts Absorption In Chemistry Adsorption — takes place on the surface of a substrate. Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. A gas absorbed by a liquid. In chemistry, absorption is a process by. Absorption In Chemistry.
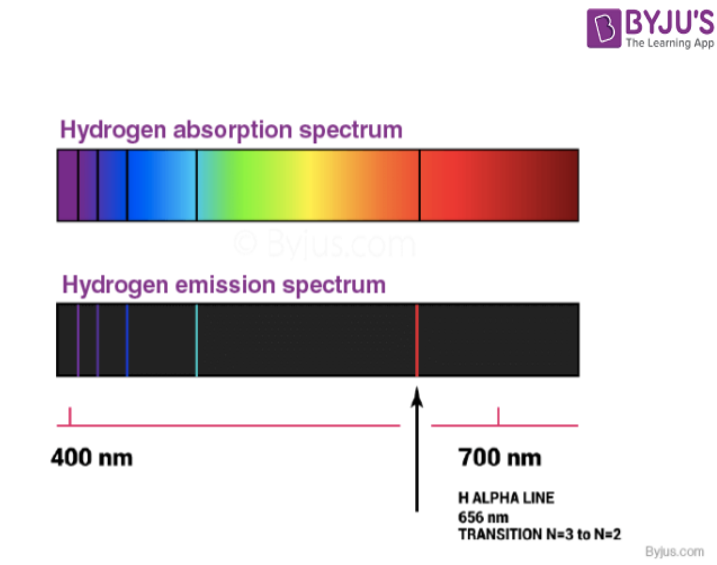
From byjus.com
Which of the following is an example of absorption? Absorption In Chemistry Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (\(\lambda\)) by a sample, and can be used to generate a spectrum, which is a plot of the absorbance as a function of the wavelength. Absorption —. Absorption In Chemistry.
From cffmleadersboard.blogspot.com
Atomic Hydrogen Absorption Spectrum Absorption In Chemistry A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (\(\lambda\)) by a sample, and can be used to generate a spectrum, which is a plot of the absorbance as a function of the wavelength. The major difference between adsorption and absorption is that one is a surface process and the other a bulk. Absorption In Chemistry.
From mavink.com
Absorption Spectrum Of Hydrogen Absorption In Chemistry Absorption differs from from adsorption, since the. Absorption — one substance enters the bulk, or volume, of another substance e.g. A gas absorbed by a liquid. Adsorption — takes place on the surface of a substrate. In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state (e.g.,.. Absorption In Chemistry.
From www.slideserve.com
PPT Physiology of absorption PowerPoint Presentation, free download Absorption In Chemistry Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state (e.g.,. The major difference between adsorption and absorption is that one is a surface process and the other a bulk. Absorption In Chemistry.
From www.youtube.com
Adsorption Vs Absorption (Differences) YouTube Absorption In Chemistry Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (\(\lambda\)) by a sample, and can be. Absorption In Chemistry.
From www.masterorganicchemistry.com
Interpreting IR Specta A Quick Guide Master Organic Chemistry Absorption In Chemistry Absorption — one substance enters the bulk, or volume, of another substance e.g. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (\(\lambda\)) by a sample, and can be used to generate a. Absorption In Chemistry.
From www.youtube.com
Atomic Absorption Spectroscopy (AAS) Explained PART 1 YouTube Absorption In Chemistry Absorption — one substance enters the bulk, or volume, of another substance e.g. Adsorption — takes place on the surface of a substrate. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. A gas absorbed by a liquid. In chemistry, absorption is a process by which a substance incorporated. Absorption In Chemistry.
From www.scribd.com
Absorption Solution Absorption (Chemistry) Absorption In Chemistry Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. Adsorption — takes place on the surface of a substrate. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength. Absorption In Chemistry.
From bisakimia.com
Kamus Kimia Absorpsi Semua Tentang Kimia Absorption In Chemistry Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state (e.g.,. Absorption differs from from adsorption, since the. A spectrophotometer in an instrument that measures the amount of light absorbed. Absorption In Chemistry.
From general.chemistrysteps.com
Rydberg Formula Chemistry Steps Absorption In Chemistry Adsorption — takes place on the surface of a substrate. A gas absorbed by a liquid. Absorption differs from from adsorption, since the. Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of. Absorption In Chemistry.
From chemicalengineeringworld.com
Gas Absorption Process Chemical Engineering World Absorption In Chemistry In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state (e.g.,. A spectrophotometer in an instrument that measures the amount of light absorbed at a specific wavelength (\(\lambda\)) by a sample, and can be used to generate a spectrum, which is a plot of the absorbance as. Absorption In Chemistry.
From scienceinfo.com
Atomic Absorption Spectrophotometry Principle, Parts, Uses Absorption In Chemistry Absorption — one substance enters the bulk, or volume, of another substance e.g. Absorption differs from from adsorption, since the. Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. In chemistry, absorption. Absorption In Chemistry.
From www.biologyonline.com
Absorption Definition and Examples Biology Online Dictionary Absorption In Chemistry Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state (e.g.,. Adsorption — takes place on the surface of a substrate. Absorption — one substance enters the bulk, or volume,. Absorption In Chemistry.
From www.researchgate.net
CO 2 capture by chemical absorption Download Scientific Diagram Absorption In Chemistry The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state (e.g.,. Absorption differs from from adsorption, since the. Absorption is the process by which atoms, molecules, or. Absorption In Chemistry.
From sciencenotes.org
Adsorption vs Absorption Differences and Examples Absorption In Chemistry Adsorption — takes place on the surface of a substrate. Absorption differs from from adsorption, since the. Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. In chemistry, absorption is a process. Absorption In Chemistry.
From www.thoughtco.com
Absorption Definition in the Chemistry Glossary Absorption In Chemistry Different materials absorb photons of different wavelengths because absorption of a photon is an absorption of energy. In chemistry, absorption is a process by which a substance incorporated in one state is transferred into another substance of a different state (e.g.,. Absorption differs from from adsorption, since the. A gas absorbed by a liquid. Absorption — one substance enters the. Absorption In Chemistry.
From www.researchgate.net
Fluorophore absorption and emission profiles. The energy of the Absorption In Chemistry Absorption differs from from adsorption, since the. Absorption is the process by which atoms, molecules, or ions enter a bulk phase (liquid, gas, solid). Adsorption — takes place on the surface of a substrate. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process. Absorption — one substance enters the. Absorption In Chemistry.